- Title
-
Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Authors
- Pillai-Kastoori, L., Wen, W., Wilson, S.G., Strachan, E., Lo-Castro, A., Fichera, M., Musumeci, S.A., Lehmann, O.J., Morris, A.C.
- Source
- Full text @ PLoS Genet.
|
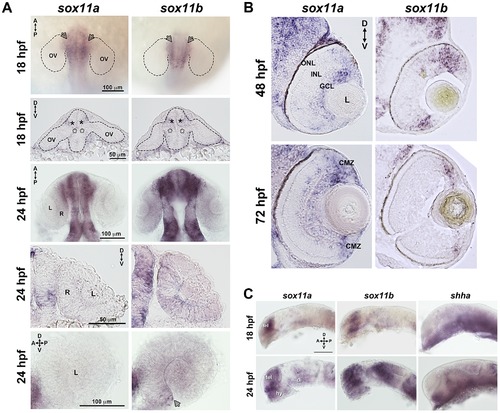
Developmental expression of sox11. In situ hybridization with antisense probes for sox11a, sox11b, and shha was performed on whole embryos or on tissue sections at the indicated time points. (A) Sox11a and sox11b were expressed in the diencephalon adjacent to the optic vesicle (arrows in top row and asterisks in second row) at 18 hpf (top two rows) and 24 hpf (third and fourth rows). Sox11a expression was not detected in the lens or retina at 24 hpf (bottom left). Sox11b was expressed in a patch of cells in the ventronasal retina (arrow, bottom right) and more diffusely across the rest of the retina and lens. Top and third rows are dorsal views of flat-mounted embryos. Second and fourth rows are frontal sections through the head. Bottom panels are lateral views of dissected eyes; (n = 20 embryos examined per time point, 3 independent repeats). (B) Transverse sections through the eye at 48 hpf (top) and 72 hpf (bottom). Sox11a expression was detected in the ganglion cell layer (GCL) and in few sporadic cells in the inner nuclear layer (INL); sox11b expression was observed in scattered cells across the central retina and in the peripheral retina. At 72 hpf, sox11a expression persisted in the GCL and in some cells in the INL; sox11a and sox11b were also expressed in the persistently neurogenic ciliary marginal zone (CMZ); n = 20 embryos examined per time point, 3 independent repeats. (C) Expression patterns of sox11a (left), sox11b (center), and shha (right) in the developing brain at 18 hpf (top) and 24 hpf (bottom). The eye was removed to better image the brain. Expression of sox11a and sox11b, but not shha, was observed in the telencephalon. Expression of all three genes was detected in the hypothalamus and ventral diencephalon at 24 hpf (n = 20 embryos examined per time point, 3 independent repeats). Scale bar = 100 μm; D, dorsal; V, ventral; A, anterior; P, posterior; hpf, hours post fertilization; OV, optic vesicle; L, lens; R, retina; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; CMZ, ciliary marginal zone; tel, telencephalon; hy, hypothalamus; di, diencephalon. EXPRESSION / LABELING:
|
|
Sox11 knockdown disrupts ocular morphogenesis and causes coloboma in zebrafish. (A) Representative eye and body images of control and sox11 morphants (taken from the set of embryos analyzed in (D). At 24 hpf, approximately 70% of sox11 morphants displayed a malformed lens (arrowhead) and a posterior kink in the tail (arrow). At 2 dpf, a similar proportion of sox11 morphants displayed coloboma (bracket), and had a hypopigmented and underdeveloped ventral retina. Both the abnormal lens and coloboma phenotypes were rescued with co-injection of wild type zebrafish sox11 mRNA (bottom row). (B) Sox11 morphants were microphthalmic at 24 hpf. Eye area was normalized to body length (*p<0.0001, Student′s t-test; control MO: n = 10 embryos examined; sox11 MO: n = 12 embryos examined, 3 independent repeats). (C) Sections of 72 hpf control (left) and sox11 morphant eyes (right) stained with cresyl violet revealed the extrusion of the retina into the brain through the open choroid fissure of sox11 morphants (asterisk); n = 6 individuals examined per group. The thickened appearance of the dorsal RPE in the sox11 morphant retina is a staining artifact and was not observed in fresh tissue sections. Scale bar = 50 µm. (D) Injection of zebrafish and human sox11 mRNA rescued the ocular phenotypes in sox11 morphants. Number of embryos analyzed: 24 hpf control MO, 4.18 ng/embryo, n = 1007; 2 dpf control MO, 4.18 ng/embryo, n = 1001; 24 hpf sox11 MO, 4.18 ng/embryo, n = 309; 2 dpf sox11 MO, 4.18 ng/embryo, n = 294; 24 hpf sox11 MO, 8.36 ng/embryo, n = 559; 2 dpf sox11 MO, 8.36 ng/embryo, n = 392; 24 hpf sox11 MO 8.36 ng/embryo plus 2.0 ng/embryo zebrafish sox11 mRNA, n = 185; 2 dpf sox11 MO, 8.36 ng/embryo plus 2.0 ng/embryo zebrafish sox11 mRNA, n = 167; 24 hpf sox11 MO, 8.36 ng/embryo plus 0.3 ng/embryo human SOX11 mRNA, n = 130; 2 dpf sox11 MO, 8.36 ng/embryo plus 0.3 ng/embryo human SOX11 mRNA, n = 125. Three biological replicates were performed for all experiments. (*p<0.001, Student′s t- test). D, dorsal; V, ventral; A, anterior; P, posterior; L, lens; R, retina; hpf, hours post fertilization; dpf, days post fertilization; MO, morpholino; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigmented epithelium. PHENOTYPE:
|
|
Sox11 morphants lack mature rod photoreceptors. (A) Representative transverse retinal sections from XOPS-GFP zebrafish injected with control, sox11 MO, or sox11 MO plus zebrafish sox11 mRNA at 3 dpf (from the set of individuals analyzed in (B). Even sox11 morphants with well-laminated retinas and no evidence of coloboma (second panel) displayed greatly reduced numbers of mature rods compared to controls (left panel). Co-injection of wild type zebrafish sox11 mRNA (right panel) rescued the rod deficiency at 3 dpf. (B) Quantification of the number of rod photoreceptors/section. Number of embryos analyzed: control MO, n = 25; sox11 MO without coloboma, n = 25; sox11 MO with coloboma, n = 25; sox11 MO plus zebrafish sox11 mRNA, n = 17 (**p<0.00001; n.s., p>0.05, Student′s t- test). (C) Two-color fluorescent in situ hybridization (FISH) for neuroD, crx, nr2e3, and rhodopsin expression in control and sox11 morphants with and without coloboma at 3 dpf. Expression of the rod lineage genes neuroD (top), crx (middle), and nr2e3 (bottom) was qualitatively normal in sox11 morphants with or without coloboma. However, rhodopsin expression (green) was greatly reduced compared to control morphants (left column). Number of embryos analyzed: n = 14 per group, 3 independent biological replicates. (D) Quantitative RT-PCR (qPCR) performed on mRNA from control and sox11 morphant heads at 3 dpf revealed a significant decrease in rhodopsin expression in sox11 morphants compared to controls. However, nr2e3 transcript levels were not significantly different between control and sox11 morphants. Relative transcript abundance was normalized to atp5h levels and is presented as the mean fold-change in expression relative to controls (n = 30 embryos per group, 3 independent biological replicates). *p<0.003; n.s, p>0.05, Student′s t-test. D, dorsal; V, ventral; L, lens; dpf, days post fertilization; MO, morpholino; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; ON, optic nerve. EXPRESSION / LABELING:
PHENOTYPE:
|
|
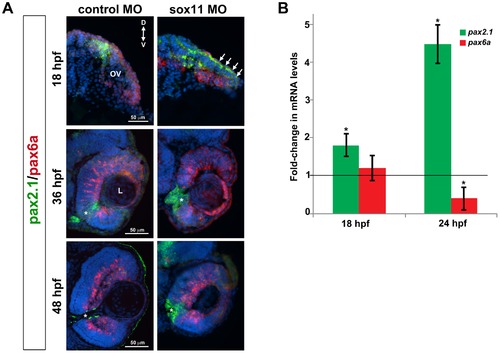
Pax2.1 and pax6a expression is altered in sox11 morphants. (A) Fluorescent in situ hybridization on transverse sections from control and sox11 morphants with probes for pax2.1 and pax6a. The expression domain of f pax2.1 was expanded into the optic vesicle of sox11 morphants at 18 hpf (top right, arrows), and there was a modest retraction of pax6a expression compared to controls (top left; number of embryos analyzed: control MO, n = 14; sox11 MO, n = 13). At 36 and 48 hpf, in control retinas pax2.1 expression decreased and was only observed lining the optic nerve (asterisk; left middle and bottom rows); in contrast, pax2.1 expression was expanded and persisted around the open choroid fissure in sox11 morphant retinas (asterisks, right middle and bottom rows). Pax6a expression in the retina of sox11 morphants at 36 and 48 hpf appeared comparable to the control morphant retinas at this stage (number of embryos analyzed: 36 hpf control MO, n = 7; 36 hpf sox11 MO, n = 12; 48 hpf control MO, n = 8; 48 hpf sox11 MO, n = 14). (B) QPCR performed on mRNA from control and sox11 morphant heads at 18 and 24 hpf revealed a significant increase in pax2.1 expression at both 18 and 24 hpf, and a downregulation of pax6a expression at 24 hpf, in sox11 morphants compared to controls. Relative transcript abundance was normalized to atp5h (18 hpf) or gapdh (24 hpf) levels and is presented as the mean fold-change in expression relative to controls (n = 50 embryos per group, 3 independent biological replicates. *p<0.05. D, dorsal; V, ventral; OV, optic vesicle; L, lens; hpf, hours post fertilization; MO, morpholino. EXPRESSION / LABELING:
|
|
Sox11 negatively regulates Hedgehog (Hh) signaling. (A) Transverse retinal sections from 24 hpf ptc2:EGFP zebrafish embryos injected with control or sox11 MO. Sox11 morphants displayed elevated GFP expression in the brain as well as in the central and dorsal retina, and the dorsal RPE (number of embryos analyzed: control MO, n = 8; sox11 MO, n = 10). (B) Treatment with the Hh inhibitor cyclopamine rescued the ocular phenotypes in sox11 morphants. In contrast, treatment with the Hh agonist purmorphamine increased the prevalence of ocular phenotypes in embryos injected with a half dose of sox11 MO. Number of embryos analyzed: 24 hpf sox11MO (plus 100% ethanol), n = 393; 2 dpf sox11 MO (plus 100% ethanol), n = 319; 24 hpf sox11 MO plus cyclopamine, n = 276; 2 dpf sox11 MO plus cyclopamine, n = 263; 24 hpf half dose sox11 MO (plus DMSO), n = 258; 2 dpf sox11 MO half dose (plus DMSO), n = 241; 24 hpf uninjected plus purmorphamine, n = 83; 2 dpf uninjected plus purmorphamine, n = 81; 24 hpf half dose sox11 MO plus purmorphamine, n = 291; 2 dpf half dose sox11 MO plus purmorphamine, n = 270; 3 independent biological replicates. * and # p<0.0001, Fisher′s exact test. (C) Treatment with cyclopamine rescued rod photoreceptor number in sox11 morphants. Rods were counted in retinal cryosections from 3 dpf embryos. Number of embryos analyzed: control MO, n = 17; sox11 MO, n = 20; sox11 MO plus cyclopamine, n = 18; 3 independent replicates. *p = 0.02, Student′s t-test). (D) Overexpression of zebrafish sox11 increased the proportion of embryos with a cyclopic phenotype (right) compared to embryos injected with equimolar amounts of control td-tomato mRNA (left). Number of embryos analyzed: control mRNA, n = 168; sox11 mRNA, n = 202, 3 independent biological replicates.*p<0.001, Fisher′s exact test. D, dorsal; V, ventral; A, anterior; P, posterior; hpf, hours post fertilization; dpf, days post fertilization R, retina; hy, hypothalamus; L, lens; MO, morpholino. EXPRESSION / LABELING:
|
|
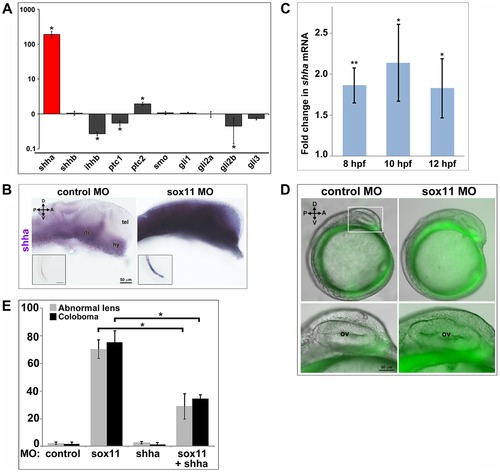
Shha expression is upregulated in sox11 morphants. (A) QPCR performed on mRNA from control and sox11 morphant heads at 24 hpf revealed a dramatic upregulation of shha expression, and a small but significant increase in ptc2 expression, in sox11 morphants compared to controls (n = 70 embryos per group, 3 independent biological replicates). Relative transcript abundance was normalized to gapdh levels. The Y-axis (log-scale) represents the mean ratio of sox11 morphant to control expression for three biological and three technical replicates. *p<0.01, Student′s t-test. (B) In situ hybridization with a shha probe on control (left) and sox11 morphant (right) embryos at 24 hpf revealed expanded shha expression in sox11 morphants throughout the brain and also in the notochord (inset). Numbers of embryos analyzed: n = 15 embryos per group, 3 independent repeats. (C) QPCR performed on mRNA from control and sox11 morphant heads at 8, 10 and 12 hpf demonstrated an upregulation of shha expression in sox11 morphants compared to controls. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 60 embryos per group, 3 independent biological repeats). **p<0.001, *p = 0.01, Student′s t-test. (D) Sox11 morphants (right) on the ptc2:EGFP background displayed elevated GFP expression in the midline at 12 hpf compared to control morphants (left). The bottom panels are an enlargement of the boxed area indicated in the top left panel. Number of embryos analyzed: control MO, n = 34; sox11 MO, n = 41, 3 independent biological replicates. (E) Co-knockdown of shha and sox11 reduced the proportion of embryos displaying abnormal lens and coloboma phenotypes at 24 hpf and 2 dpf, respectively. Number of embryos analyzed: 24 hpf control MO, n = 186; 2 dpf control MO, n = 165; 24 hpf sox11 MO, n = 199, 2 dpf sox11 MO, n = 182; 24 hpf shha MO, n = 249; 2 dpf shha MO, n = 231; 24 hpf sox11+ shha MO, n = 207; 2 dpf sox11+ shha MO, n = 190; 3 independent biological replicates. *p<0.0001, Student′s t-test. D, dorsal; V, ventral; A, anterior; P, posterior; hpf, hours post fertilization; dpf, days post fertilization; R, retina; di; diencephalon, tel, telencephalon; hy, hypothalamus; MO, morpholino. EXPRESSION / LABELING:
|
|
Bmp7b expression is reduced in sox11 morphants. (A) QPCR was performed on mRNA from control and sox11 morphant heads at 24 hpf for known repressors of shha transcription. A significant downregulation of bmp7b was observed in sox11 morphants compared to controls. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 50 embryos per group, 3 independent biological repeats). **p<0.01, Student′s t ?test. (B) Injection of bmp7b mRNA significantly reduced the proportion of sox11 morphants displaying abnormal lens and coloboma phenotypes at 24 hpf and 2 dpf, respectively. Number of embryos analyzed: 24 hpf control MO, n = 127; 2 dpf control MO, n = 123; 24 hpf sox11 MO, n = 282; 2 dpf sox11 MO, n = 274; 24 hpf bmp7b mRNA, n = 95; 2 dpf bmp7b mRNA, n = 91; 24 hpf sox11 MO + bmp7b mRNA, n = 140, 2 dpf sox11 MO + bmp7b mRNA, n = 134; 3 independent biological replicates. *p<0.006. (C) Brightfield images of a representative sox11 morphant and a sox11 morphant rescued with bmp7b mRNA, taken from the set of embryos analyzed in (B). D, dorsal; V, ventral; A, anterior; P, posterior; hpf, hours post fertilization; dpf, days post fertilization; MO, morpholino. |
|
Efficiency and specificity of sox11 morpholinos. (A) Schematic representation of the pEF1α:GFP plasmid containing a portion of the sox11 52 UTR placed upstream of the GFP reporter (top). The binding site for the sox11 morpholino is shown in red. Separate reporters were constructed for the sox11a and sox11b MOs. (Center) Lateral view (anterior at top) of 24 hpf embryos injected with EF1α- sox11a/b-GFP plasmids alone (left) or with both sox11 MOs. No GFP expression was detected in the embryo injected with sox11 MOs. (Bottom) Quantification of the proportion of GFP-positive embryos at 24 hpf. The sox11 MOs were highly effective at blocking GFP expression. Number of embryos analyzed: pEF1α-sox11-GFP plasmid alone, n = 169; pEF1α-sox11-GFP + sox11 MOs, n = 140, 3 independent repeats; *p = 0.004, Student′s t-test. (B) Both sox11a and sox11b contribute to abnormal lens and coloboma phenotypes observed in sox11 morphants. The proportion of embryos displaying either phenotype was significantly higher when injected with sox11a and sox11b MOs simultaneously, compared to either MO alone. Number of embryos analyzed: 24 hpf control MO, n = 463; 2 dpf control MO, n = 441; 24 hpf sox11a MO, n = 229; 2 dpf sox11a MO, n = 214; 24 hpf sox11b MO, n = 341; 2 dpf sox11b MO, n = 316; 24 hpf sox11a + sox11b MO, n = 271; 2 dpf sox11a + sox11b MO, n = 262, 3 independent repeats. *p<0.001, Fisher′s exact test. (C) A second non-overlapping sox11 MO (that targeted both sox11a and sox11b simultaneously) produced the same coloboma phenotype in similar proportion to the first set. Number of embryos analyzed: control MO, n = 186 embryos; sox11 MO, n = 194, 3 independent repeats; *p<0.001, Fisher′s exact test. MO, morpholino; hpf, hours post fertilization; dpf, days post fertilization. |
|
Cell proliferation and apoptosis in sox11 morphants. (A) Quantification of TUNEL+ cells in the optic vesicle, lens, and retina of control and sox11 and morphants from 18?72 hpf. Sox11 morphants had an elevated number of TUNEL+ cells in the optic vesicle at 18 hpf. Additionally, sox11 morphants consistently displayed more TUNEL+ cells in the anterior lens compared to controls from 24 -72 hpf. Number of embryos analyzed: 18 hpf control MO, n = 20; 18 hpf sox11 MO, n = 22; 24 hpf control MO, n = 15; 24 hpf sox11 MO, n = 19; 48 hpf control MO, n = 10; 48 hpf sox11 MO, n = 13; 72 hpf control MO, n = 12; 72 hpf sox11 MO, n = 12; average of 3 independent biological replicates. **p<0.00001, *p<0.01, Student′s t-test. (B) Representative transverse sections of control (left column) and sox11 (right column) morphants at 18, 24, and 48 hpf, taken from the set of individuals analyzed in (A). At 48 hpf, TUNEL+ cells were detected within the colobomatous tissue and the region of the optic stalk in sox11 morphants (arrow, bottom right). (C) Sox11 morphant retinas had more PH3+ cells than controls from 18?72 hpf. Number of embryos analyzed: 18 hpf control MO, n = 12; 18 hpf sox11 MO, n = 15; 24 hpf control MO, n = 20; 24 hpf sox11 MO, n = 19; 48 hpf control MO, n = 10; 48 hpf sox11 MO, n = 12; 72 hpf control MO, n = 14; 72 hpf sox11 MO, n = 12; average of 3 independent biological replicates. **p<0.001, *p<0.01, Student′s t-test. (D) Representative transverse sections of control (left column) and sox11 (right column) morphants at 18, 24, and 48 hpf, taken from the set of individuals analyzed in (C). D, dorsal; V, ventral; MO, morpholino; hpf, hours post fertilization; ON; optic nerve; OV, optic vesicle; R, retina; L, lens. PHENOTYPE:
|
|
Retinal neurogenesis in sox11 morphants. (A) Retinal cell types were visualized by immunohistochemistry (ganglion, amacrine, horizontal, and bipolar cells) or with fluorescent reporter transgenic lines (Tg(gfap:GFP)mi2001 for Müller glia and Tg(3.2TαC-EGFP) for cones) in controls (left) and sox11 morphants (center, right) at 3 dpf. In sox11 morphants without coloboma (center), the retinas are well laminated and had normal numbers of ganglion, amacrine, horizontal, and bipolar cells, cone photoreceptors, and Müller glia. However, sox11 morphants with coloboma (asterisk; right) had poorly laminated retinas and reduced numbers of differentiated retinal cell types, indicating delayed retinal development. (B) Quantification of numbers of late-born retinal cell types in control and sox11 morphants without coloboma. Only rod photoreceptors displayed a significant reduction. Number of embryos analyzed: control MO, n = 19; sox11 MO without coloboma, n = 25, 3 independent repeats.**p<0.00001; ns = p>0.05, Student′s t-test.(C) At 4 dpf, sox11 morphants have more mature rod photoreceptors than at 3 dpf but the number remains significantly less than controls (*p<0.001, Student′s t-test); MO, morpholino; dpf; days post fertilization; L, lens; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; ON, optic nerve. |
|
Elevated Hh signaling contributes to the abnormal ocular phenotypes displayed by sox11 morphants. (A) Representative brightfield images of sox11 morphants treated with cyclopamine, purmorphamine and their corresponding vehicle controls at 24 hpf and 2 dpf, taken from the set of embryos analyzed in Figure 5. Treatment with 75 uM purmorphamine alone did not cause any abnormalities (last column; 24 hpf control MO plus 75 μM purmorphamine alone, n = 123; 2 dpf control MO plus 75 μM purmorphamine alone, n = 114, 3 independent biological repeats). (B) Suppression of Hh pathway with cyclopamine rescued the rod photoreceptor defect in sox11 morphants at 3 dpf (right; number of embryos analyzed: sox11 MO, n = 20; sox11 MO + cyclopamine, n = 18; 3 independent repeats). (C) Retinal cell types were visualized by immunohistochemistry (ganglion, cones, amacrine, horizontal, and bipolar cells) or with a transgenic fluorescent reporter lines (Tg(gfap: GFP)mi2001) for Müller glia in sox11 morphants (left) and sox11 morphants treated with cyclopamine (right) at 3 dpf. The retinas of sox11 morphants treated with cyclopamine were well laminated and displayed normal distributions of all cell types (n = 15 per group, 3 independent repeats). D, dorsal; V, ventral; A, anterior; P, posterior; MO, morpholino; hpf, hours post fertilization; L, lens. |
|
Hh pathway gene expression changes in sox11 morphants. (A) QPCR performed on mRNA from sox11 morphant and control heads at 18 hpf reveal small increases in gli2a and gli3 expression in sox11 morphants compared to controls, but no significant change in shha expression. Relative transcript abundance was normalized to atp5h levels and is presented as the mean fold-change in expression relative to controls (B) At 24 hpf, sox11 morphants demonstrated a large increase in shha expression, which correlated with the dose of sox11 MO injected. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 60 embryos per group, 3 independent biological repeats) *p<0.01, Student′s t-test. (C) Representative bright-field images of embryos injected with sox11 MO alone (left side), shha MO alone (middle), or both shha and sox11 MOs (right side), taken from the set of embryos analyzed in Figure 6E. (D) Co-knockdown of shha increased rod photoreceptor number in sox11 morphants at 3 dpf (number of embryos analyzed: control MO, n = 11; shha MO, n = 10; sox11 MO, n = 16, sox11+ shha MO, n = 15, 3 independent repeats) *p<0.05, Student′s t-test. (E) QPCR performed on mRNA from heads of sox11 morphants treated with vehicle (100% ethanol) or cyclopamine and compared to control morphants treated with vehicle revealed a significant reduction in shha expression in sox11 morphants treated with cyclopamine at 24 hpf. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 40 embryos per group, 3 independent biological repeats) **p<0.0001, Student′s t-test. (F) QPCR for shha was performed on mRNA from the 24 hpf heads of sox11 morphants injected with half the normal dose and treated with DMSO, sox11 morphants (half dose) treated with purmorphamine, and compared to control morphants treated with DMSO. An increase in shha expression was detected in sox11 morphants (half dose) treated with purmorphamine compared to sox11 morphants (half dose) treated with DMSO, however the increase did not reach the threshold for statistical significance. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 40 embryos per group, 3 independent biological repeats). (G) QPCR was performed on mRNA from the 24 hpf heads of zebrafish embryos injected with control (td-tomato) mRNA and embryos injected with zebrafish sox11 mRNA. This analysis revealed a significant decrease in shha expression in embryos overexpressing zebrafish sox11 mRNA compared to the controls. Relative transcript abundance was normalized to 18s rRNA levels and is presented as the mean fold-change in expression relative to controls (n = 30 embryos per group, 3 independent biological repeats). **p<0.0001, Student′s t-test. (H) QPCR performed on mRNA from heads of 24 hpf embryos injected with shha MO or control MO revealed no significant change in expression of either sox11a or sox11b in shha morphants compared to controls. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 45 embryos per group, 3 independent biological repeats). ns, p>0.05, Student′s t-test. D, dorsal; V, ventral; A, anterior; P, posterior; MO, morpholino; hpf, hours post fertilization; dpf, days post fertilization; L, lens. |
|
Sox4 compensates for the loss of Sox11. (A) Sox4a was diffusely expressed in the control retina at 36 hpf (left); however, sox4a expression was upregulated in the lens and retina of sox11 morphants (right; n = 20 per group); scale bar = 50 µm. (B) QPCR performed on mRNA from the heads of 24 hpf zebrafish embryos injected with sox11 MO or control MO reveal that sox4a expression is elevated in sox11 morphants compared to controls. Relative transcript abundance was normalized to gapdh levels and is presented as the mean fold-change in expression relative to controls (n = 40 embryos per group, 3 independent biological repeats) *p<0.01, Student′s t-test. (C) Co-injection of sox4 mRNA rescued the lens and coloboma phenotypes of sox11 morphants at 24 hpf and 2dpf. Number of embryos analyzed: 24 hpf control MO, n = 136; 2 dpf control MO, n = 124; 24 hpf sox11 MO, n = 179; 2 dpf sox11 MO, n = 161, 24 hpf sox11 MO + sox4 mRNA, n = 210, 2 dpf sox11 MO + sox4 mRNA, n = 184, 3 independent biological replicates. *p<0.001, Fishers exact test; MO, morpholino. |
|
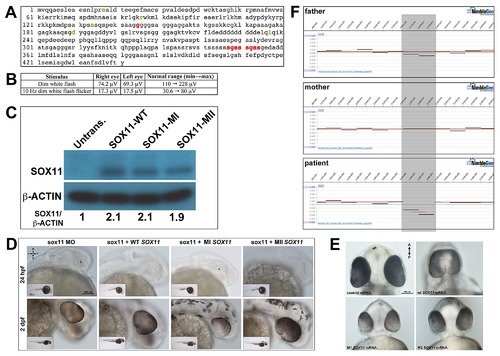
Association of SOX11 locus with ocular abnormalities. (A) Amino acid sequence of human SOX11, with previously identified non-synonomous SNPs highlighted in green. The two variants identified in the MAC patients (positions indicated in red) are novel. (B) Scotopic ERG analysis of the proband′s mother carrying the S315?354dup variant, demonstrating a reduction in the b-wave amplitude. (C) Western blot for SOX11 and β-actin in COS-7 cells transfected with SOX11 expression constructs. Densitometric analysis was performed with ImageJ software. (D) Representative brightfield images of sox11 morphants co-injected with either WT, MI (G145C), or MII (S315?354dup) SOX11 mRNA at 24 hpf and 2 dpf, taken from the set of embryos analyzed in Figure 8D. (E) Representative brightfield images of embryos overexpressing human WT, MI, or MII SOX11 mRNA, taken from the set of embryos analyzed in Figure 8F. (F) Array CGH analysis of a proband with optic nerve agenesis and microphthalmia and her parents, confirming the presence of a de novo interstitial deletion at chromosome 2p25.2 (shaded gray). D, dorsal; V, ventral; A, anterior; P, posterior; MO, morpholino; hpf, hours post fertilization; dpf, days post fertilization; L, lens. |