- Title
-
V-ATPase proton pumping activity is required for adult zebrafish appendage regeneration
- Authors
- Monteiro, J., Aires, R., Becker, J.D., Jacinto, A., Certal, A.C., Rodríguez-León, J.
- Source
- Full text @ PLoS One
|
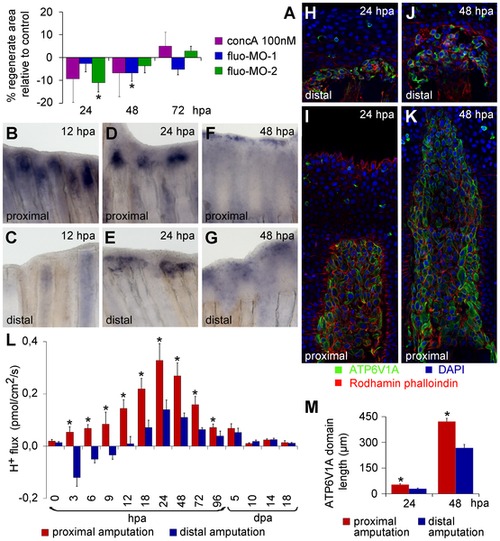
(A) Recording chamber for H+ flux measurement using SIET, representing zebrafish emerged in recording medium. (B) H+ efflux established during regeneration. *statistical significant results (n = 10, p<0.05). (C) Affimetrix microarray to assess gene expression during caudal fin regeneration, compared to intact fins. (D?I) In situ hybridization of V-ATPase subunits atp6v1e1b (D?F) and atp6v1a (G?I), at 24 hpa (D, G), 48 hpa (E?H) and 72 hpa (F?I). (J?M) Immunohistochemical detection of Atp6v1a at 24 hpa (J, K), 48 hpa (L) and 72 hpa (M). White arrowheads point to the Atp6v1a blastema localization. (J) Detail of Atp6v1a (green) cellular localization in blastema cells, 24 hpa. hpa: hours post amputation. For each panel, n = 6, except mentioned otherwise. EXPRESSION / LABELING:
|
|
V-ATPase inhibition affects regeneration and, similar to H+ efflux, has a different expression pattern depending on the amputation plane along the PD axis. (A) Inhibition of the V-ATPase using concanamycinA (concA) and fluorescein- tagged morpholinos (fluo-MO-1 and 2). (B?M) Experiments performed after proximo-distal amputation. (B?G) In situ hybridization for atp6v1e1b at 12 h (B, C), 24 h (D, E) and 48 h (F, G) after proximal (B, D, F) and distal amputation (C, E, G). (H?K) Immunohistochemical detection of Atp6v1a at 24 h (H, I) and 48 h (J, K) after proximal (I, K) and distal amputation (H, J). (L) H+ efflux pattern during regeneration after proximal and distal amputation (n = 8?10). (M) Length of Atp6v1a expression domain in proximal and distal stumps, 24 and 48 hpa. *statistical significant results (n = 3, p<0.05). hpa: hours post amputation. For each panel, n = 6, except mentioned otherwise. EXPRESSION / LABELING:
|
|
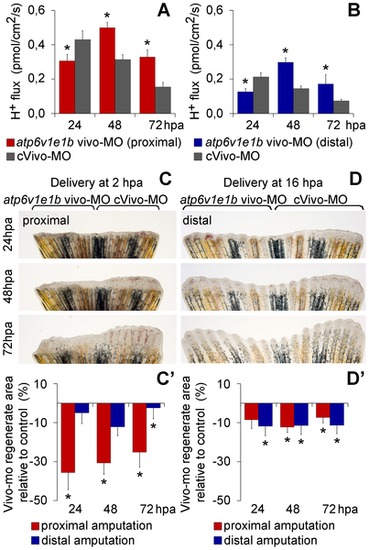
Vivo-morpholino mediated atp6v1e1b knockdown impairs regeneration and decreases H+ flux. (A, B) H+ flux during regeneration after vivo-MO mediated atp6v1e1b knockdown, 2 h after proximal (A) and distal (B) amputation. *statistical significant results (p<0.05). (C, D) Caudal fin regeneration after atp6v1e1b vivo-MO delivery to atp6v1e1bhi577aTg/+ (AB) fish, 2 h after proximal amputation (C) and 16 h after distal amputation (D). (C′, D′) Percentage of regenerate area in atp6v1e1b knocked down regions compared to the control vivo-MO (cVivo-MO). Vivo-MOs were delivered 2 h (C′) or 16 h (D′) after both proximal and distal amputation. hpa: hours post amputation. For each panel, n = 8. PHENOTYPE:
|
|
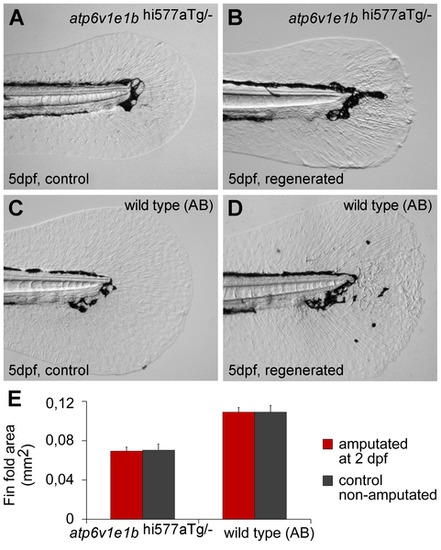
V-ATPase is not required for larval fin fold regeneration. (A, B) Fin fold of 5 dpf atp6v1e1hi577aTg/- mutant larvae, intact (A) or regenerated after amputation at 2 dpf (B). (C, D) Fin fold of 5 dpf AB wild type larvae, intact (C) or regenerated after amputation at 2 dpf (D). (E) Area of intact and regenerated fin fold by 5 dpf for either genotypes (p>0.05). dpa: days post amputation. For each panel, n = 15. |
|
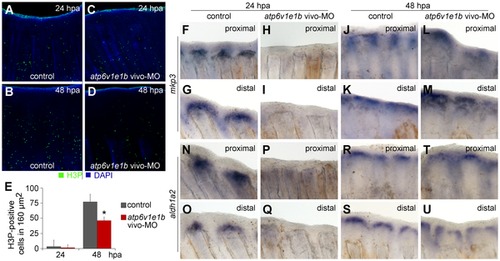
V-ATPase is required for blastema cell proliferation and expression of mkp3 and aldh1a2. (A?D) Immunohistochemical detection of proliferating cells (H3P-positive) 24 h (A, C) and 48 h (B, D) after proximal amputation. (A, B) control fin; (C, D) V-ATPase knocked down fin. (E) Quantification of H3P-positive cells in the blastema. *statistical significant results (p<0.05). (F?U) In situ hybridization for mkp3 (F-M) and aldh1a2 (N-U), after proximal amputation (F, H, J, L; N, P, R, T) and distal amputation (G, I, K, M; O, Q, S, U): (F?G; N?O) control fin, 24 hpa; (H?I; P?Q) V-ATPase knocked down fin, 24 hpa; (J?K; R?S) control fin, 48 hpa; (L?M; T?U) V-ATPase knocked down fin, 48 hpa. Control fin: caudal fin from AB wild type fish, non-treated. V-ATPase knockdown procedure: atp6v1e1b vivo-MO delivered to the caudal fin of atp6v1e1bhi577aTg/+ (AB) fish, 2 hpa. hpa: hours post amputation. For each panel, n = 3. |
|
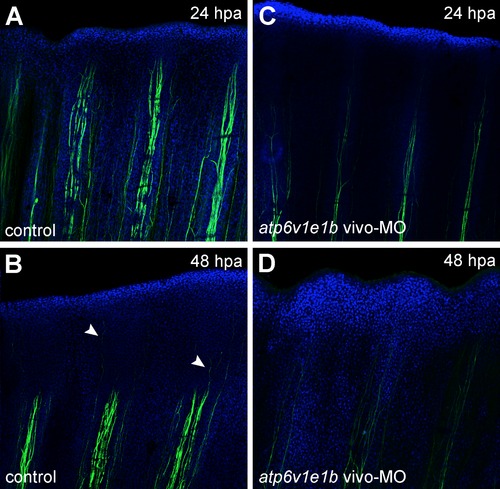
V-ATPase inhibition affects fin innervation. Immunohistochemical detection of acetylated α-tubulin (axons marker) under control regeneration conditions (A, B) and after atp6v1e1b knockdown, 2 h after proximal amputation (C, D). (A,C) 24 hpa, (B, D) 48 hpa. Arrow heads: innervation of regenerating tissue. hpa: hours post amputation. For each panel, n = 3. |
|
V-ATPase subunits localization in chloride cells, intact and regenerating fins. Whole mount in situ hybridization for atp6v1e1b (Figure A) and atp6v1a (Figure B) in intact fins. Cross section of the whole mount in situ hybridization for atp6v1a at 24 hours post amputation (hpa) where expression can be observed in the blastema, distal to the bone (Figure C, arrowheads). Atp6v1a in the intact caudal fin is present mainly in the epidermis, in a scattered pattern (Figure D). Whole mount in situ hybridization (Figure E) and immunostaining (Figure F) for atp6v1a subunit in the chloride cells of the zebrafish embryo. |
|
Delivery of control and atp6v1e1b fluorescein-tagged morpholinos. Merged fluorescent and bright field image showing the incorporation of both control and atp6v1e1b fluorescein-tagged morpholinos into opposite regions of a regenerating fin, 24 h after delivery. |
|
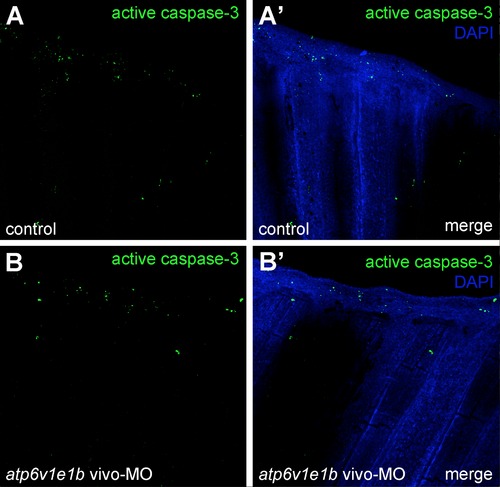
V-ATPase inhibition does not affect apoptosis during regeneration. atp6v1e1b knockdown didn′t affect cell proliferation by 24 hpa compared to the control (compare Figures A-A′ with B-B′). |
|
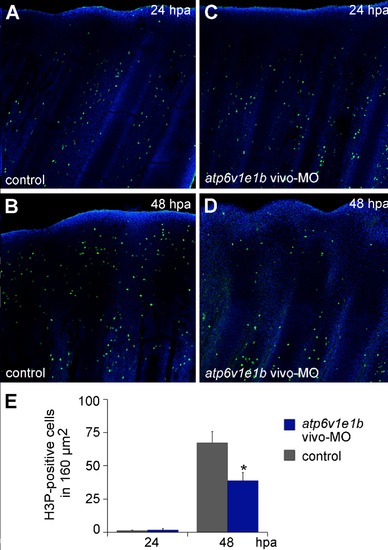
V-ATPase is required for cell proliferation in the mature blastema after amputation at the distal plane. atp6v1e1b knockdown didn′t affect cell proliferation by 24 hpa (compare Figures A and C; E). However, at 48 hpa there were significantly less blastema cells positive for H3P than in the control (compare Figures B and D; E). |
|
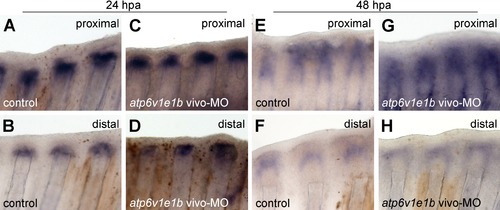
Expression of wnt10a is not affected by V-ATPase knockdown. In situ hybridization for wnt10a showed a stronger/wider expression in proximal stumps than in distal ones, both at 24 and 48 hpa under normal regeneration conditions (compare Figures A and B, Figures E and F). This pattern remained unchanged upon atp6v1e1b knockdown (compare Figures C and D, Figures G and H). |
|
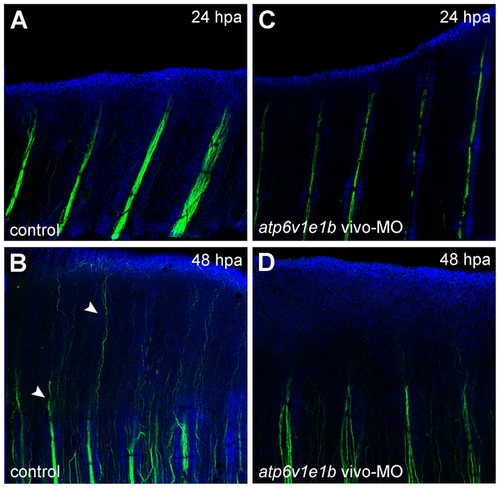
V-ATPase inhibition affects fin innervation after distal amputation. Fins were immunostained for acetylated α-tubulin under normal regeneration conditions (control) and after atp6v1e1b knockdown at 2 hpa. V-ATPase knockdown decreased fin innervation both below the amputation plane and in the regenerating tissue (compare Figures A-B with C-D, respectively). |