- Title
-
The titin A-band rod domain is dispensable for initial thick filament assembly in zebrafish
- Authors
- Myhre, J.L., Hills, J.A., Prill, K., Wohlgemuth, S.L., and Pilgrim, D.B.
- Source
- Full text @ Dev. Biol.
|
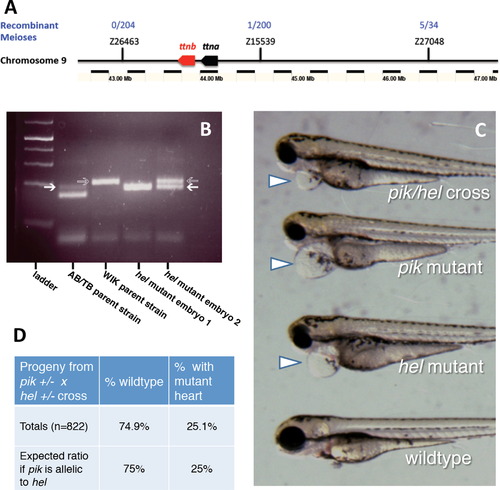
The herzschlag mutation is an allele of the ttna gene. (A) Microsatellite mapping with SSLP markers on zebrafish chromosome 9 shows a linkage between the herzschlag mutant locus and a ~1 Mbp region including the titin gene cassette. Flanking markers showed minimal recombination between the AB/TB and WIK polymorphic backgrounds in ~200 meioses. (B) Resolution of SSLP markers used for microsatellite mapping. AB/TB genetic markers (white arrows) migrate faster than WIK markers (gray arrows), and thus a double band indicates a recombination event between the marker and the mutation, while a single lower band indicates no recombination. (C) The outcome of a complementation cross between herzschlag and the known heart-isoform-specific ttna mutant pickwick demonstrates that the two mutations fail to complement. The phenotype of the heteroallelic mutant (top) is indistinguishable from the pickwick mutant phenotype (pik), displaying pericardial edema, reduced heartbeat, small head and eyes, but with normal locomotion. The complementation cross produced this mutant phenotype with the expected Mendelian ratios (D), whether hel males and pik females were used, or vice versa (206 embryos out of n=822 total offspring from four individual crosses). PHENOTYPE:
|
|
The mutant phenotype of herzschlag is characterized by circulation and edema-related defects, shortened myofibers and disorganized sarcomeres. Comparison of the hel mutant with other motility mutants (steif) at 3 days post-fertilization (dpf) demonstrates the common phenotypes (A), including heart edema (arrowheads), small head and eyes, and reduced organization of trunk musculature (arrows). Reduced heartbeat in motility mutants results in pericardial edema and dysplasia of muscular (B and C, upper panels) and skeletal (lower panels) elements of the head by 5 dpf. Compared with wild-type embryos (B), hel mutants (C) have smaller occulomotor muscles (arrows), laterally displaced interhyoideus muscles (arrowheads) and ceratohyal cartilages (lower panel, arrowheads), and reduced or absent hyoideus muscles. DIC imaging of somites in 5 dpf hel embryos (F) shows a complete loss of myocyte striations as compared to WT (arrow in D), with shortened myofibers separated by vacuole-like spaces (arrow in E). A?P indicates the direction of the anterior?posterior axis. Immunostaining for thick filament myosin (F) and thin filament actin (G) at 3 dpf demonstrates the extent of myofibril disorganization, with greatly shortened myofibers and lateral separations between cells. (H) A merged image shows co-localization of myosin and actin in mutant embryos. At higher magnification (I and J), it can be seen that some myosin periodicity (arrow) seems to remain in hel mutants (bars in I), but at a much shorter period than in wild-type embryos (bars in J). The integrity of myosepta was not affected by the hel mutation (arrowheads in E and F). This is particularly evident following immunostaining of extracellular laminin in WT (K) and hel mutant embryos (L). In all experiments, a minimum of 20 embryos were examined to ensure consistency of the phenotype. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The herzschlag mutation results in a form of titin that fails to cross-react with antibodies specific for the thick filament rod domain. Immunofluorescent staining with antibodies directed against specific peptides of the titin protein shows that mutant embryos lack the T11 peptide, found at the I-band to A-band transition, but retain the T12 peptide, which localizes just lateral to the Z-disk (represented schematically in panel A). A?P indicates the direction of the anterior?posterior axis. Whole-mount WT hel siblings (B, D, F, and H) and hel mutant embryos (C, E, G, and I) were identified phenotypically at 30 hpf and stained with antibodies against these respective peptides (green in B?E). Embryos were also counter-stained with fluorescently labeled phalloidin to show actin organization (red in F?I). Anti-T11 antibody staining was clearly visible in WT embryos (B and F) but not in herzschlag mutants (C and G). By contrast, the anti-T12 staining was clearly visible in both WT (D and H) and hel mutant embryos (E and I), localizing to the Z-disk as expected (arrow), despite apparent disorganization of the myofibrils (E and I). In all experiments, a minimum of 20 embryos were examined to ensure consistency of the phenotype. Artwork by Alina Pete. |
|
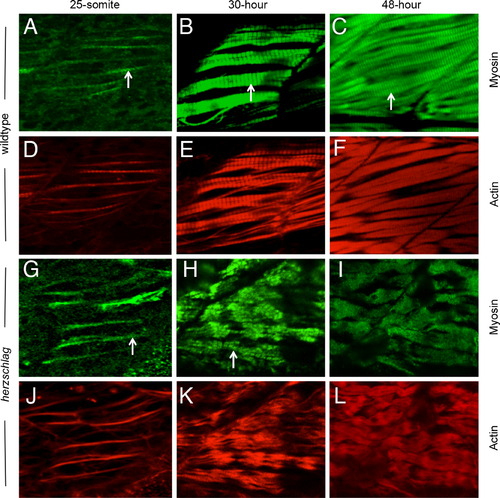
Myosin thick filament organization still occurs in the absence of the titin rod domain. Herzschlag mutants and wild-type embryos were followed through a time-course of myogenesis, and stained at 24, 30 and 48 hpf with antibodies against slow-muscle myosin (green). In wild-type embryos (A?F), the striations of organized thick filaments can be seen at all time-points throughout myofibrillogenesis (arrows in A?C). Counter-staining with fluorescently labeled phalloidin to show actin organization (red) reveals a similar pattern of thin filament organization (D?F). In hel mutant embryos, the striated pattern of myosin thick filaments was retained at 24 h (G, arrow). By 30 hpf, myofibers were clearly disorganized (H), but retained a striated pattern of thick filament myosin (arrow). This pattern was only lost completely after 48 hpf (I). Thin filament striations, as show by phalloidin counter-staining, were never visible at any timepoint in hel mutant embryos (J?L). In all experiments, a minimum of 20 embryos were examined to ensure consistency of the phenotype. EXPRESSION / LABELING:
PHENOTYPE:
|
|
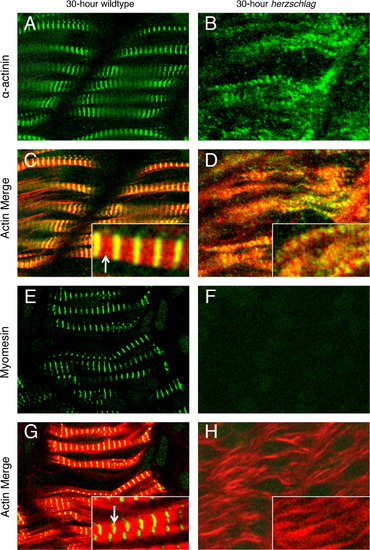
Localization of myomesin but not α-actinin is lost in the titin truncation mutant. Wild-type (left) and herzschlag mutant embryos (right) were examined at 30 hpf to determine the effects of titin truncation on the organization of specific regions of the sarcomere. Integrity of the Z-disk was assessed by immunofluorescent labeling of α-actinin (A and B), while integrity of the M-line was assessed by labeling of myomesin (E and F). All markers can be seen in their normal arrangement in WT embryos when merged with a phalloidin counter-staining to show actin (red), which leaves a narrow gap at the M-line (arrows). Narrow bands of green fluorescence correspond to the Z-disk for α-actinin (C, inset) and the M-line for myomesin (G, inset). In hel embryos (right), expression of α-actinin was detected (B), and sarcomere organization was reduced as in Fig. 5, with α-actinin striations detectable in some myofibers (D, inset). By contrast, myomesin localization was not detectable in hel mutant embryos (F and H). In all experiments, a minimum of 20 embryos were examined to ensure consistency of the phenotype. |
|
Stress-responsive gene expression is not increased in hel mutant embryos during early myofibrillogenesis. Expression of myosin folding stress-responsive genes hsp90a1 (A?D) and unc45b (E?H) did not change in hel mutant embryos (C and D and G and H) when compared with wild-type controls (A and B and E and F). Increased mRNA expression of stress-responsive genes was assessed by in situ hybridization at 24 (left) and 36 hpf (right). At 24 hpf, when increased expression of myosin chaperones is expected in mutants with myosin assembly defects, no qualitative change could be detected between WT and hel mutant embryos for either gene (compare A with C and E with G). Even at 36 hpf, expression was not greatly increased (compare B with D and F with H), although the extent of mRNA expression in the tail somites was somewhat greater in mutants (arrows). There was little variability between individuals within experimental groups from two independent experiments (n=20 embryos per group). |
|
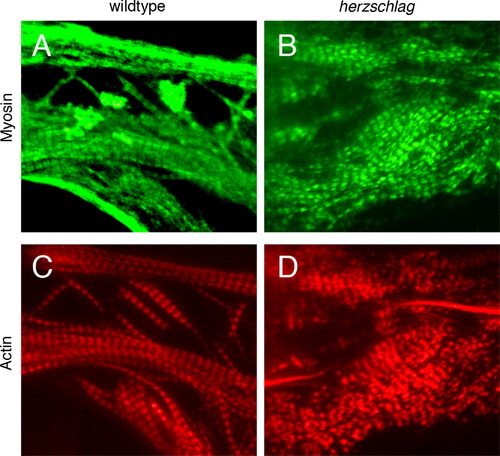
hel mutant myocytes that are not subject to contractile signals in culture retain sarcomere organization. Dissociated blastomeres from individual WT (left) and hel mutant (right) embryos were cultured on laminin substrate to induce myogenesis for 5 days prior to fixation. Individual cultures were genotyped by PCR to confirm the identity of mutants and siblings. Immunofluorescent staining for myosin (green, A and B) and actin (red, C and D) demonstrated relatively normal sarcomere patterning in mutants as compared with WT (compare banding patterns in A with B and in C with D), with only a mild effect on sarcomere organization in mutants, although myosin A-band patterning with a standard sarcomere period could be readily discerned (B and D). There was little variability between cultures of the same time-point (n=64 total cultures examined). |
Reprinted from Developmental Biology, 387(1), Myhre, J.L., Hills, J.A., Prill, K., Wohlgemuth, S.L., and Pilgrim, D.B., The titin A-band rod domain is dispensable for initial thick filament assembly in zebrafish, 93-108, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.