- Title
-
The Critical Role of Protein Arginine Methyltransferase prmt8 in Zebrafish Embryonic and Neural Development Is Non-Redundant with Its Paralogue prmt1
- Authors
- Lin, Y.L., Tsai, Y.J., Liu, Y.F., Cheng, Y.C., Hung, C.M., Lee, Y.J., Pan, H., and Li, C.
- Source
- Full text @ PLoS One
|
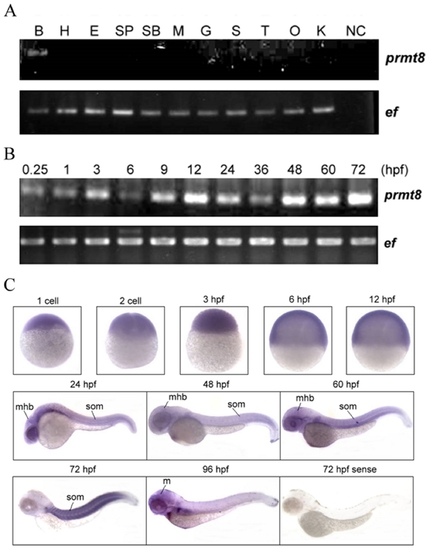
Expression analysis of prmt8 RNA in zebrafish adult tissues and embryos. (A) Expression of prmt8 in different adult tissues was analyzed by RT-PCR. EF indicates the RT-PCR product of elongation factor. (B: brain; SP: spleen; G: gill; O: ovary; H: heart; SB: swim bladder; S: skin; E: eye; M: muscle; T: testis) (B) RT-PCR of prmt8 RNA prepared from 0.25 hpf to 72 hpf embryos. (C) The spatial and temporal expression of prmt8 RNA from 1 cell to 96 hpf by WISH in zebrafish embryonic development. (m: midbrain; Mhb: mid-hindbrain boundary; som: somites). |
|
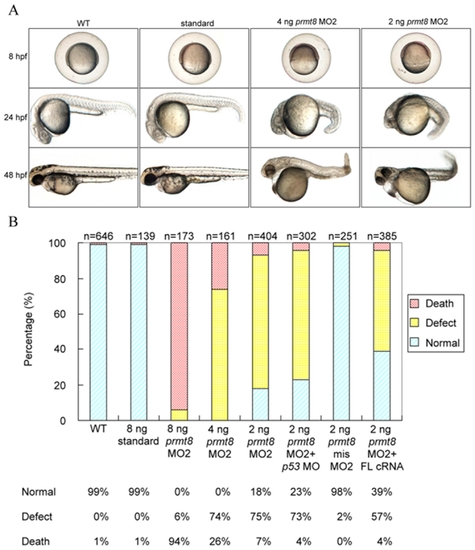
Phenotypes of zebrafish Prmt8 morphants. (A) Phenotypes of zebrafish embryos not injected (WT; wildtype), injected with control AMO (standard), or injected with 2 or 4 ng prmt8 MO2 at 8, 24, and 48 hpf. (B) Percentage of normal, dead, or defective zebrafish embryos not injected, injected with standard AMO, prmt8 MO2 (2, 4 and 8 ng), 2 ng of MO2 co-injected with p53 MO, 5- mispair MO2, or 2 ng of MO2 co-injected with full-length prmt8 cRNA. The embryos were examined at 24 hpf. Embryos with any of the gross phenotypic changes including curved tails, small and malformed brain and eyes, short yolk stalk, enlarged and swollen yolk and cardiac edema were classified as “defect”. PHENOTYPE:
|
|
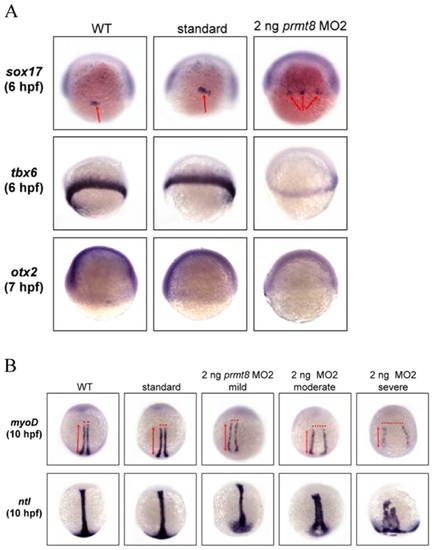
Defective phenotypes at gastrulation and early segmentation stage of the zebrafish prmt8 morphants. (A) Zebrafish embryos not injected (WT), injected with control AMO (standard) or 2 ng of prmt8 MO2 were stained with an endodermal marker sox 17 (animal pole views) or a mesodermal marker tbx6 (side views) at 6 hpf, and an ectodermal marker otx2 at 7 hpf. (B) Zebrafish embryos not injected, injected with control AMO or 2 ng of prmt8 MO2 were stained with myoD or ntl at 11 hpf (dorsal views). |
|
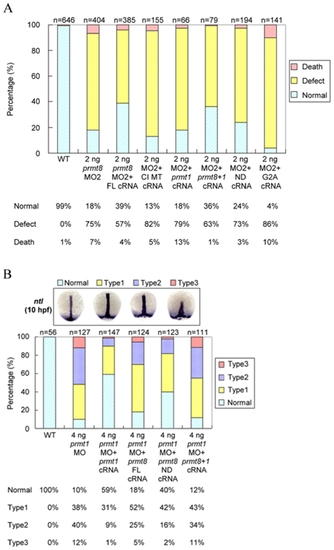
Rescue of prmt8 or prmt1 morphants with prmt8 or prmt1 cRNA. (A) Frequencies of normal, dead or defective embryos not injected (WT), injected with 2 ng of prmt8 MO or prmt8 MO with full-length prmt8 (FL) cRNA, catalytic inactive mutant prmt8 (CI MT) cRNA, full-length prmt1 cRNA, prmt8 N-terminus with full-length prmt1 (prmt8+1) cRNA, N-terminus-deleted prmt8 (ND) cRNA, or G2A mutated prmt8 cRNA. The embryos were examined and classified as described in Figure 3B. (B) Frequencies of different phenotypes of embryos not injected (WT), injected with 2 ng of prmt1 MO, or prmt1 MO with full-length (FL) prmt1 cRNA, full-length prmt8 cRNA, N-terminal deleted (ND) prmt8 or prmt8 N-terminus with full-length prmt1 (prmt8+1) cRNA Injected embryos were stained with ntl at 10 hpf and the morphants were grouped according to the degree of shortening and widening of the notochord. |
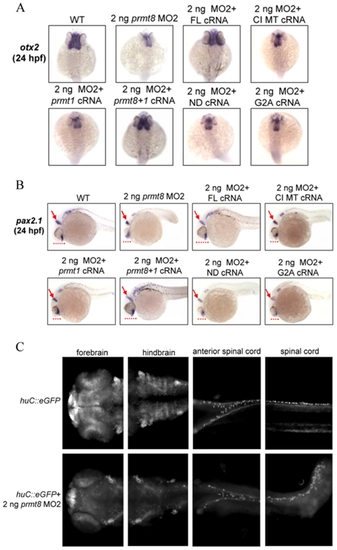
|
Characterization of perturbed brain phenotypes in the prmt8 morphants. Zebrafish embryos (24 hpf) injected with 2 ng of prmt8 MO2, or 2 ng of MO2 together with full-length (FL) prmt8 cRNA, prmt8 N-terminus with full-length prmt1 (prmt8+1) cRNA, N-terminus-deleted (ND) prmt8 cRNA, catalytically inactive mutant (CI MT) prmt8 cRNA or G2A mutated prmt8 cRNA, were stained with otx2 (A; dorsal view with anterior to the top) or pax2.1 (B; lateral view with anterior to the left). Arrows indicate aberrant expression patterns at the mid-hindbrain boundaries in morphant embryos. Dash lines indicate the relative length of the dorsoventral axis of the developing brain. (C) Typical embryos from the Tg(huC::eGFP) fish, images were taken by Leica DM2500 epifluorescence microscope with a DFC490 CCD camera. |
|
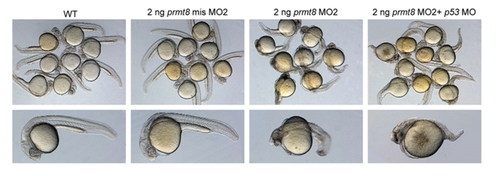
Knockdown of p53 did not rescue the defects of prmt8 morphant. Zebrafish embryos injected with 2 ng of MO2 were co-injected with p53 MO or not. Similar defects and defect rates were observed at 24 hpf in both population. PHENOTYPE:
|

Validation of the inhibitory efficiency of the prmt8 morpholino. Embryos were injected with a vector expressing Prmt8-GFP fusion protein or the vector together with MO2 and then examined by fluorescent microscopy. |
|
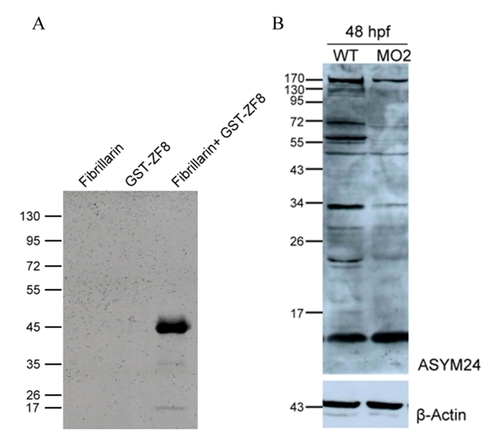
Type I protein arginine methyltransferase activity of zebrafish Prmt8. (A) GST-fused recombinant zebrafish Prmt8 was expressed in Escherichia coli and purified. In vitro methylation was conducted with Prmt8 and recombinant mouse fibrillarin as the methyl-accepting protein in the presence of 1.5 μCi of [methyl-3H]-AdoMet (60 Ci/mmol, Amersham Biotech) at 37°C for 60 min in a total volume of 15 μl in reaction buffer (50 mM sodium phosphate, pH 7.5). The samples were subjected to SDS-PAGE. The gels were then stained, treated with EN3HANCE (Perkin Elmer) and dried for fluorography. Control reactions with methyl-accepting protein (fibrillarin) or methyltransferase (GST-ZF8) only were conducted. (B) Reduced asymmetric dimethylarginine polypeptide signals in zebrafish prmt8 morphants. Embryos injected with 2 ng of MO2 (MO) or not (WT) were collected at 48 hpf. Fifty microgram of embryonic extract protein was subjected to western blot analyses with an asymmetric dimethylarginine-specific antibody (ASYM24). β-actin was used as a loading control. |
|
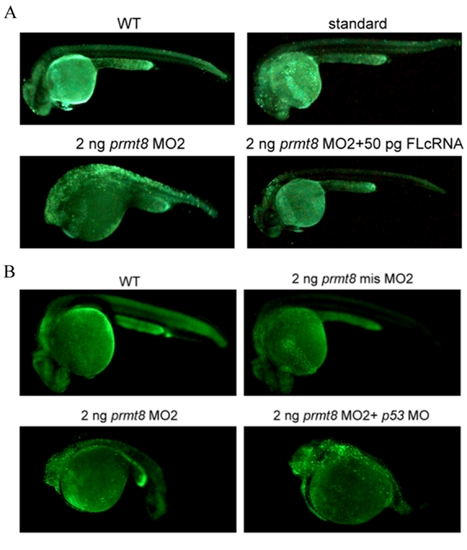
Knockdown of prmt8 leads to apoptosis in embryos. Apoptosis analysis of zebrafish embryos at 24 hpf were conducted by acridine orange stain. (A) Embryos not injected, injected with standard AMO, injected with 2 ng prmt8 MO2 or 2 ng prmt8 MO2 and full-length prmt8 cRNA are shown. (B) Embryos not injected, injected with 2 ng prmt8 MO2, or 2 ng prmt8 MO2 and p53 MO are shown. PHENOTYPE:
|