- Title
-
Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue
- Authors
- Deng, Q., Yoo, S.K., Cavnar, P.J., Green, J.M., and Huttenlocher, A.
- Source
- Full text @ Dev. Cell
|
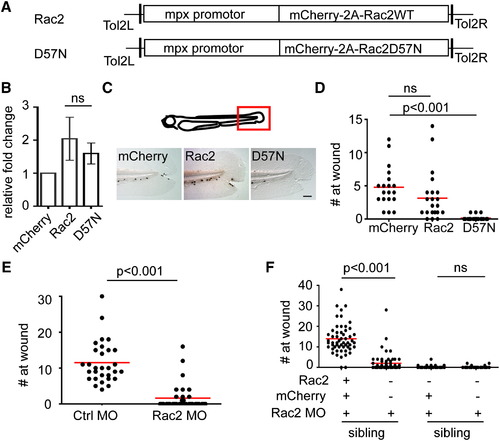
Neutrophil Intrinsic Rac2 Activity Is Required for Neutrophil Wound Response (A) Schematics of Tol2-MPX:mCherry-2A-zRac2 vectors, both wild-type and D57N, injected into wild-type AB zebrafish to generate Tg(mpx:mCherry-2A-Rac2) (Rac2) and Tg(mpx:mCherry-2A-Rac2D57N) (D57N) transgenic lines. (B) Relative expression level of Rac2 mRNA in neutrophils in Rac2 and D57N lines compared with Tg(mpx:mCherry)(mcherry) determined by quantitative RT-PCR. Result is presented as mean ± SD (n = 3). Ns, nonsignificant, two-tailed paired t test. (C) Sudan black staining of neutrophils at tail wounds at 1 hr post wounding (hpw) in 3 dpf mCherry, Rac2, or D57N larvae. Scale bar represents 50 μm. (D) Quantification of neutrophil wound response shown in (C). n = 35 (mCherry), 37 (Rac1), and 42 (D57N). p < 0.001, Kruskal-Wallis test followed by Dunn′s multiple comparison test. (E) Morpholino oligonucleotides (MO)-mediated knockdown of Rac2 reduced neutrophil recruitment to tail wound at 2hpw. n = 30 (ctrl MO) and 37 (Rac2 MO). p < 0.001, two-tailed Mann-Whitney U test. (F) Expression of MO-resistant mCherry-2A-zRac2WT, but not mCherry alone, in neutrophils rescues defect in wound recruitment in Rac2 morphants. Columns 1-2 and 3-4 are siblings, which are used as an internal control for MO efficacy. n = 55, 60, 54, and 40 for columns 1<4. p < 0.001, Kruskal-Wallis test followed by Dunn′s multiple comparison test. See also Figure S1 and Movie S1. PHENOTYPE:
|
|
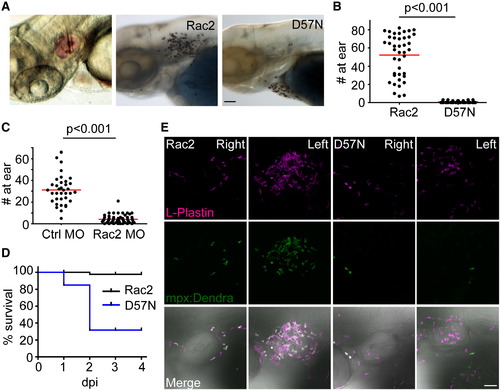
Rac2D57N-Expressing Zebrafish Larvae Are More Susceptible to Bacterial Infection (A) Left, demonstration of zebrafish larvae otic infection. Phenol red tracking dye (pink) was used to visualize bacterial infection. Sudan black staining of neutrophil recruitment 2 hr post ear infection (hpi) in Rac2 (middle) and D57N larvae (right) larvae. Scale bar represents 50 μm. (B) Neutrophils recruited to the ear 2 hpi were quantified. n = 41 (Rac2) and 39 (D57N). p < 0.001, two-tailed Mann-Whitney U test. (C) Neutrophils recruited to the ear 1 hpi were quantified in control and Rac2 morphants. n = 36 (Ctrl MO) and 57 (Rac2 MO). p < 0.001, two-tailed Mann-Whitney U test. (D) Survival of larvae challenged with <5 × 104 cfu of PAK (pMKB1::mCherry). n = 16 (Rac2) and 18 (D57N). (E) Recruitment of leukocytes to otic infection in Rac2 and D57N larvae. Zebrafish larvae from Tg(mpx:dendra2) crossed with Rac2 or D57N lines were infected with PAK (<104 cfu) into the left ear. Total leukocyte populations were immunostained with L-Plastin antibody. Neutrophils were visualized by Dendra2 green fluorescence. The right ear of the same fish was shown as control. Images are representative for at least 15 individual fish. Scale bar represents 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
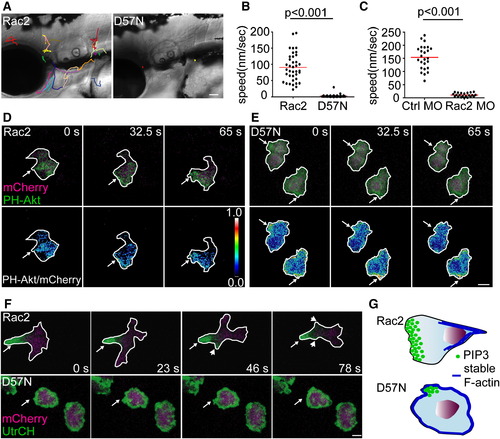
Rac2 Activity Is Required for Neutrophil Motility and Polarity In Vivo (A) Tracking of neutrophil random migration in the head in Rac2 and D57N larvae (lateral view). Scale bar represents 50 μm. (B) Quantification of 3D speed of neutrophil random motility in mesenchymal tissues of the head of Rac2 or D57N larvae. n = 38 (Rac2) and 45 (D57N). p < 0.001, two-tailed Mann-Whitney U test. (C) Quantification of 3D speed of neutrophil random motility in mesenchymal tissues in the head of Ctrl and Rac2 morphants. n = 25 (Ctrl MO) and 22 (Rac MO). p < 0.001, two-tailed Mann-Whitney U test. (D and E) Tg(mpx:GFP-PH-Akt) was crossed to Rac2 (D) and D57N (E) lines to visualize the localization of PI(3,4,5)P3-PI(3,4)2 in neutrophils randomly migrating in mesenchymal tissues of the head. Ratiometric images were generated using mCherry as a volumetric control. Still images from representative movies of randomly migrating neutrophils in the head are shown. Arrows, pseudopods in (D) and protrusions in (E). (F) Tg(mpx:GFP-UtrCH) was crossed to Rac2 and D57N lines to visualize the localization of stable F-actin. Still images from representative movies of randomly-migrating neutrophils in the head are shown. Upper, arrow, uropod; arrowhead, retracting pseudopods. Lower, arrow, protrusion. Scale bar represents 5 μm (D-F). (G) Schematics of altered cell polarity in D57N neutrophils. PI(3,4,5)P3-PI(3,4)2 primarily localizes to the pseudopods in Rac2 neutrophils. D57N neutrophils form transient protrusions that are enriched for PI(3,4,5)P3-PI(3,4)2, but fail to form functional pseudopods. Stable F-actin is polarized and enriched in the uropod in Rac2 neutrophils but is present in protrusions and is not polarized in D57N neutrophils. See also Figure S2, Movie S2, Movie S3, Movie S4, and Movie S5. PHENOTYPE:
|
|
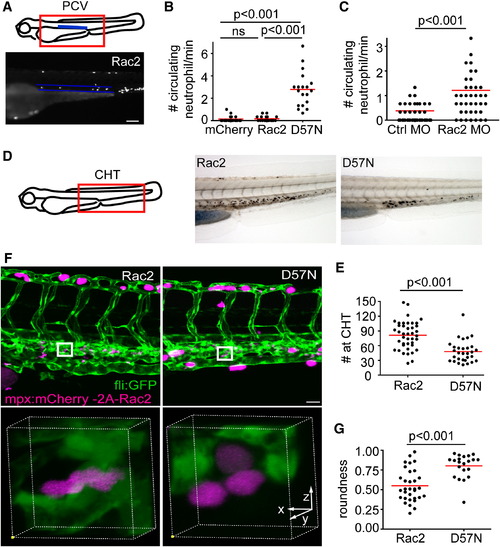
Neutrophilia in Rac2-Deficient Larvae (A) Illustration of the location of the posterior cardinal vein (PCV) that was imaged for quantification of circulating neutrophils. Neutrophils that circulate through the PCV per minute were scored. Lower, one still image from the representative movie of Rac2 larvae containing circulating neutrophils within the highlighted PCV (blue lines). Scale bar represents 100 μm. (B) Quantification of neutrophils that circulate through the PCV per min in mCherry, Rac2, and D57N larvae at 3 dpf. n = 20 each for mCherry, Rac2, and D57N larvae. p < 0.001, Kruskal-Wallis test followed by Dunn′s multiple comparison test. (C) Quantification of neutrophils that circulate through the PCV per min in control or Rac2 morphants at 2 dpf. n = 40 each for Ctrl or Rac2 morphants. p < 0.001, two-tailed Mann-Whitney U test. (D) Sudan black staining of neutrophils in the CHT. (E) Quantification of neutrophil number in the CHT of Rac2 and D57N larvae at 3 dpf. n = 41 (Rac2) and 30 (D57N). p < 0.001, two-tailed Mann-Whitney U test. (F) Three-dimensional volume rendering of neutrophil morphology in the CHT in Rac2 and D57N larvae. Boxed regions are enlarged in the lower panel. Founder fish are crossed with Tg(fli1:GFP) to visualize endothelial tissues or stroma. Scale bar represents 30 μm. (G) Quantification of cell roundness in the CHT in Rac2 and D57N larvae. n = 32 (Rac2) and 23 (D57N). p < 0.001, two-tailed Mann-Whitney U test. See also Figure S3 and Movie S6. PHENOTYPE:
|
|
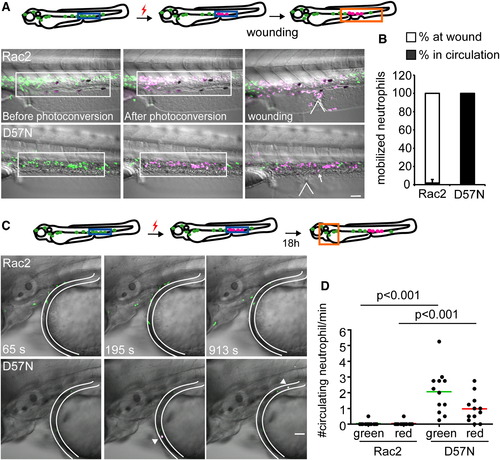
Rac2D57N Results in Increased Release of Neutrophils from the CHT (A) Rac2 or D57N lines were crossed with Tg(mpx:dendra2). Photoactivation converts green fluorescent Dendra2 to red, allowing for fate-mapping 1.5 hr postventral fin wounding. Neutrophils in boxed region (CHT) were photoconverted. Locations of wounds were indicated with the wedged lines. Arrow, a circulating neutrophil. Scale bar represents 50 μm. (B) Distribution of photolabeled neutrophils that are mobilized out of the CHT in Rac2 and D57N larvae 2 hpw. Open bar, % of photolabeled neutrophils at wound; filled bar, % of photolabeled neutrophils in circulation. n = 6 larvae for each condition, results are presented with mean ± SD. (C) Neutrophils from the CHT in 2 dpf D57N larvae spontaneously entered the circulation 18 hr after photolabeling. Still images from representative movies in the head are shown. Location of blood vessel is delineated. Arrow, circulating photoconverted neutrophils. Scale bar represents 50 μm. (D) Quantification of (C). Green and red represent total and photolabeled circulating neutrophils, respectively. n = 12 each. p < 0.001, Kruskal-Wallis test followed by Dunn′s multiple comparison test. See also Figure S4 and Movie S7. PHENOTYPE:
|
|
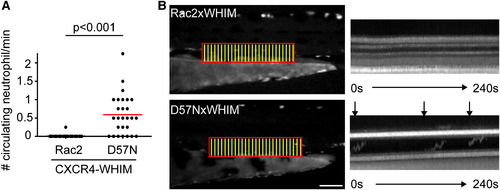
Rac2D57N Partially Rescues CXCR4-Mediated Retention of Neutrophils in the CHT (A) Tg(mpx:CXCR4-WHIM) was crossed with Rac2 or D57N lines and circulating neutrophils were quantified. n = 26 each. p < 0.001, two-tailed Mann-Whitney U test. (B) Fluorescent signal in boxed region was stacked vertically into one-dimensional line at each time point. Kymography of 4-min movies with 2-s interval is shown. Horizontal lines indicate auto-fluorescent pigments that are stationary throughout the movie. Arrows indicate neutrophils circulating in the PCV. Scale bar represents 100 μm. See also Figure S5. PHENOTYPE:
|
|
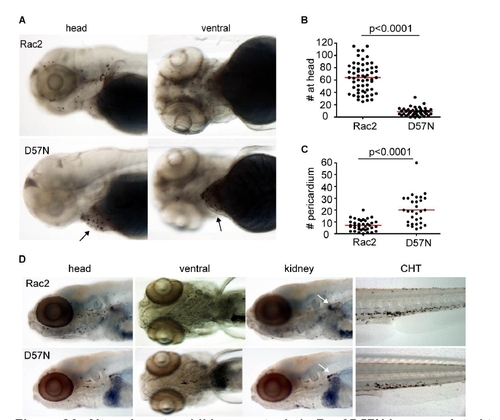
Altered neutrophil homeostasis in Rac2D57N larvae, related to Figure 4. (A) in 3dpf larvae, compared to Rac2, D57N transgenic larvae have reduced neutrophil number in the head. Arrows point to the pericardium region where most neutrophils are present in D57N larvae. (B,C,) Quantification of (A). n=58 (Rac2), 46 (D57N) in (B) and n=38 (Rac2), 31 (D57N) in (C). p<0.0001, two-tailed Mann-Whitney test. (D) Altered neutrophil homeostasis persists at 7dpf. Note, there are fewer neutrophils in kidney in D57N larvae as well. CHT, caudal hematopoietic tissue. |
|
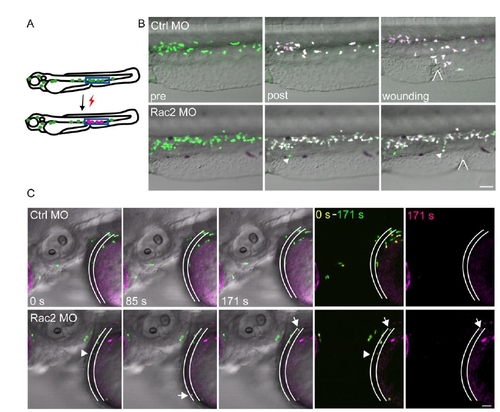
Figure S4. Fate-mapping of neutrophils from CHT, related to Figure 5. (A) Schematics of photoconversion. UV converts green fluorescent Dendra2 to red, allowing for fate-mapping. (B) Photolabeled neutrophils responded to the wound in a control morphant, whereas in a Rac2 morphant, neutrophils did not migrate to the wound, but entered circulation. Locations of wounds were highlighted. Arrow, circulating neutrophil. Images are representative for 6 larvae from 2 separate experiments. Scale bar, 50 μm. (C) Neutrophil in the CHT at 2dpf Rac2 morphants spontaneously entered circulation 18 h after photo-conversion. Still images from one movie representative for 9 zebrasfish (3 independent experiments) are shown. Location of blood vessel is highlighted. Arrow, circulating photoconverted neutrophils. Arrow head, a circulating non-photoconverted neutrophil. Scale bar, 30 μm. |
|
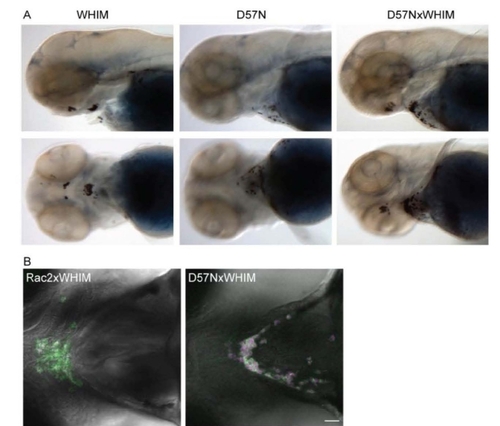
Figure S5. Rac2 D57N partially inhibited CXCR4-WHIM induced neutrophil clustering, related to Figure 6. (A) Sudan black staining of neutrophil clusters at the ventral side of the head where SDF-1 is expressed in Tg(mpx:CXCR4-WHIM). Rac2 D57N expression in neutrophil partially inhibits this clustering. (B) Confocal images of neutrophil distribution at the ventral side of the head in Tg(mpx:CXCR4-WHIM, mpx:mCherry-2A-Rac2 WT or D57N) at 3dpf. Green, CXCR4-GFP; red, Rac2-2A-mCherry. Scale bar, 30 μm. |
Reprinted from Developmental Cell, 21(4), Deng, Q., Yoo, S.K., Cavnar, P.J., Green, J.M., and Huttenlocher, A., Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue, 735-745, Copyright (2011) with permission from Elsevier. Full text @ Dev. Cell