- Title
-
nev (cyfip2) Is required for retinal lamination and axon guidance in the zebrafish retinotectal system
- Authors
- Pittman, A.J., Gaynes, J.A., and Chien, C.B.
- Source
- Full text @ Dev. Biol.
|
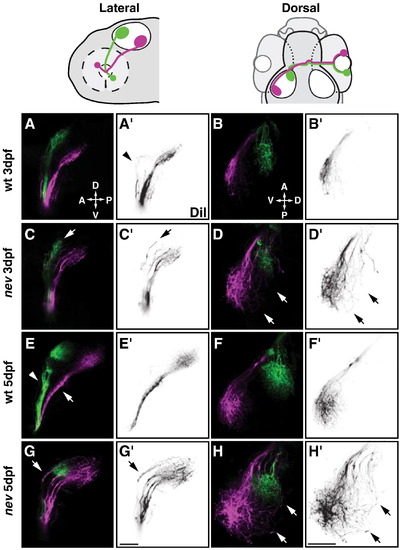
nevermind/cyfip2 is required for axon sorting and targeting of dorsonasal retinal axons. Confocal projections of dorsonasal axons (in magenta) and ventrotemporal axons (in green) in WT (A, B, E, F) and nev (C, D, G, H) at 3dpf (A?D) and 5dpf (E?H). (A′?H′) show only DiI injected dorsonasal axons in reverse contrast. Cartoons above show orientation of lateral views (A, C, E, G) and dorsal views (B, D, F, H). WT axons (A and E) from dorsonasal and ventrotemporal retina are topographically sorted in the ventral branch (arrow in E) and dorsal branch (arrowhead in E) of the optic tract, respectively. Arrowhead in A′ shows a few dorsal axons in WT projecting in the dorsal branch but turning before entering the tectum. Once on the tectum, WT axons project topographically to their target (B and F). However, in nev, dorsonasal axons are missorted in the dorsal branch of the optic tract (arrows in C and G), and project through the dorsal half of the optic tectum (arrows in D and H). Scale bars = 50 μm. PHENOTYPE:
|
|
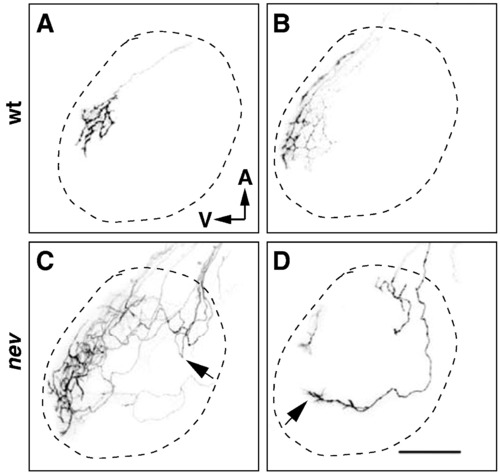
Dorsonasal axons take circuitous routes on the tectum in nev. (A?D) Confocal images of the left optic tectum after DiI labeling of a few RGCs in the dorsal retina of wt (A and B) or nev (C and D) at 5dpf. Dashed line indicates approximate outline of tectum. In WT, dorsonasal axons enter the tectum through the ventral branch of the optic tract, project directly to their topographic target and arborize (A and B). In nev, dorsonasal axons that enter through the dorsal branch of the optic tract meander around the dorsal tectum, sometimes turning back anteriorly after entering the tectum (arrow in C) but appear to orient toward the ventral tectum (arrow in D). Scale bar in D = 50 μm. PHENOTYPE:
|
|
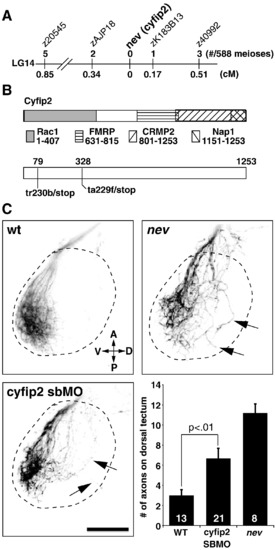
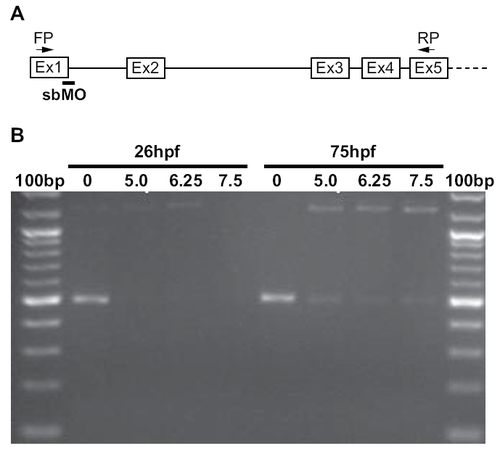
nevermind encodes Cyfip2. (A) Meiotic map of nev/cyfip2. Using a combination of SSLPs and SNPs, nev was fine-mapped to a 0.51 cM interval on LG14. Numbers above show recombinations in 588 meioses; numbers below indicate distance in cM. (B) Structure of Cyfip2. Domains known to be important for protein interactions with Cyfip1 and Cyfip2 are shown (see text for references). cDNA sequencing from tr230b and ta229f mutant embryos identified premature stop codons 79 amino acids (tr230b) and 328 amino acids (ta229f) into the protein. (C) A cyfip2 splice-blocking morpholino phenocopies nev. Dorsal views showing dorsonasal axons projecting onto the optic tectum. Dashed lines indicate approximate outline of the tectum. Dorsonasal axons in nev project inappropriately onto the dorsal half of the optic tectum, as do dorsonasal axons in embryos injected with a splice-blocking morpholino (SBMO) to cyfip2 (arrows). To quantify these errors, the number of axons projecting through the dorsal optic tectum was counted in wt, cyfip2 SBMO, and nev at 3dpf. Numbers of embryos shown inside bars. Scale bar = 50 μm. PHENOTYPE:
|
|
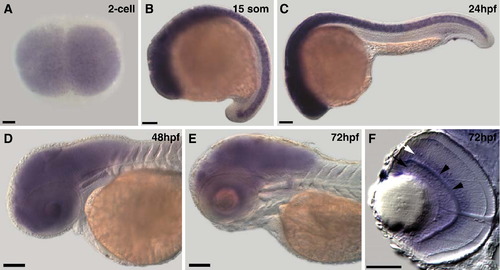
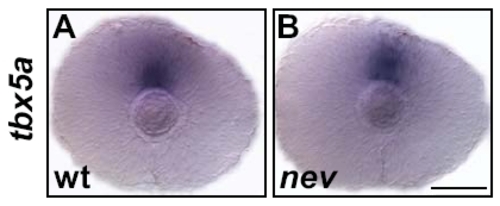
cyfip2 is broadly expressed in the CNS. (A) Animal pole view of a two-cell embryo showing maternal expression of cyfip2. (B and C) Lateral views of a 15 somite and 24hpf embryo, respectively. cyfip2 is broadly expressed in the CNS. (D and E) From 48hpf to 72hpf, when axons first arrive at the optic tectum and begin to innervate it, cyfip2 is still broadly expressed in the brain and retina. (F) Coronal section through a 72hpf eye. cyfip2 is expressed in the RGC layer (black arrow), the inner plexiform layer (black arrowheads), and the inner nuclear layer (white arrow). Scale bars = 100 μm (A?E), 50 μm (F). EXPRESSION / LABELING:
|
|
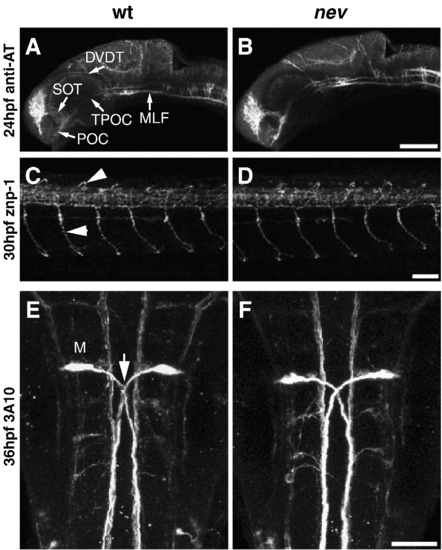
Early axon pathfinding appears normal in nev. Confocal projections in WT (A, C, E) and nev (B, D, F) stained with anti-acetylated tubulin (A and B), znp-1 (B and E), and 3A10 (C and F). Lateral views (A?D) and dorsal views (E and F). Anti-acetylated tubulin labels early axon pathways at 24hpf including the anterior commissure (AC), post-optic commissure (POC), supra-optic tract (SOT), tract of the post-optic commissure (TPOC), dorso-ventral diencephalic tract (DVDT), and medial longitudinal fasciculus (MLF). No differences are detectable between WT (A) and nev (B). Axon trajectories of primary motor neurons at 30hpf are normal in nev (D) compared to WT (C), including middle primary motor neurons (MiPs, upper arrowhead) and caudal primary motor neurons (CaPs, lower arrowhead). (E and F) Mauthner neurons and their axons (arrow in E) project normally in nev (F) as compared to WT (E) at 36hpf. Scale bar in B = 100 μm, D, F = 50 μm. |
|
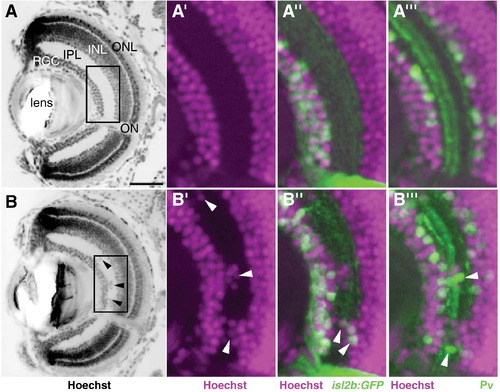
Retinal lamination is disrupted in nev. Coronal sections through a WT eye (A?A″′) and a nev eye (B?B″′) at 5dpf. (A and B) Hoechst 33342 stain to visualize lamination. The retinal ganglion cell (RGC) layer, inner plexiform layer (IPL), inner nuclear layer (INL), and outer nuclear layer (ONL) are all clearly visible at this stage. Arrowheads in B show displaced cells in the IPL of nev. Boxed regions are magnified in A′?A″′ and B′?B″′ and show nuclei labeled with Hoechst 33342 (magenta; A′?A″′, B′?B″′), RGCs labeled with isl2b:GFP (green; A″, B″), and a subset of amacrine cells labeled with anti-parvalbumin (green; A″′, B″′). In nev, RGCs and amacrine cells are intermingled in the IPL. Arrowheads in B″ and B″′ show displaced RGCs and amacrine cells in the IPL, respectively. ON, optic nerve. Scale bar = 50 μm. |
|
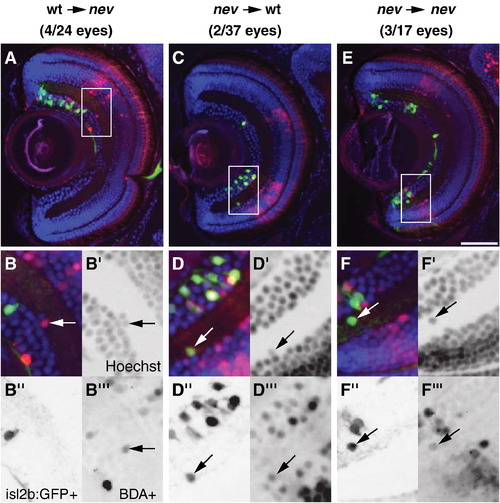
cyfip2 acts both cell autonomously and cell nonautonomously in lamination. Representative coronal sections through a nev eye with WT donor cells (A?B″′), a WT eye with nev donor cells (C?D″′), and a nev eye with nev donor cells (E-F″′). (A, C, E) Hoechst 33342 stain (blue), biotin dextran-positive (BDA+) donor cells (red), and isl2b:GFP+ donor RGCs (green). Insets are shown magnified below. (B?B″′) WT donor cells in a nev host are misplaced in the IPL, showing nev can act cell nonautonomously. Arrows show a BDA+ donor cell next to a BDA cell in the IPL. (D?D″′) nev donor cells in a WT host are misplaced in the IPL, showing that nev can also act cell autonomously. Arrows show a BDA+/isl2b:GFP+ cell in the IPL. (F?F″′) Control transplants show nev donor cells in a nev host that are misplaced in the IPL. Arrow shows a BDA+/isl2b:GFP+ cell misplaced in the IPL. Scale bar = 50 μm. |
|
cyfip2 acts cell autonomously in dorsonasal RGC axon sorting in the optic tract. (A) Co-electroporation yields consistent co-labeling with EGFP and mCherry. (B) Diagram of rescue experiments: electroporated dorsonasal RGCs project to the posteroventral quadrant of the tectum. (C) Quantification of D-V sorting of dorsonasal axons in the optic tract as they enter the right optic tectum, relative to the ventralmost (0%) and dorsalmost (100%) fascicles (white ruler in D). A subset of nev axons missorts into the dorsal brachium; electroporation with cyfip2 yields significant rescue. Error bars show mean ± SEM. (D?F) Confocal projections of single dorsonasal axons (GAP43-GFP, green) in the tectal neuropil (mCherryCAAX, red) at 5 dpf. (D) WT axons enter the tectum ventrally. (E) nev axons often enter dorsally. (F) cyfip2-co-electroporated nev axons are rescued and enter ventrally. Scale bar, 50 μm. PHENOTYPE:
|
|
|
|
|
Reprinted from Developmental Biology, 344(2), Pittman, A.J., Gaynes, J.A., and Chien, C.B., nev (cyfip2) Is required for retinal lamination and axon guidance in the zebrafish retinotectal system, 784-794, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.