- Title
-
prep1.2 and aldh1a2 participate to a positive loop required for branchial arches development in zebrafish
- Authors
- Vaccari, E., Deflorian, G., Bernardi, E., Pauls, S., Tiso, N., Bortolussi, M., and Argenton, F.
- Source
- Full text @ Dev. Biol.
|
Expression of prep1.2 during development. A, B: RT-PCR analysis showing amplification of prep1.2 from morula to 48 hpf stages. C?J: Whole-mount in situ hybridization experiments with a prep1.2 antisense probe showing that prep1.2 is weakly and ubiquitously expressed from 128 cells to tailbud stage (panels from C to F). G: during somitogenesis prep1.2 becomes more concentrated in cells localized on each side of the neural tube. H: At 24 hpf prep1.2 transcripts remain weakly ubiquitous with stronger expression in the CNS (forebrain and midbrain), eyes and in cell clusters localized on each side of the NS, posterior to the otic vesicle. I: at 36 hpf prep1.2 is expressed in the head and in the pharyngeal and pectoral fin bud mesenchyme. J: From 48 hpf onwards prep1.2 expression decreases and is restricted to the head region. e: eye; h: hindbrain; m: midbrain; nt: neural tube; ov: otic vesicle; pba: prospective branchial arches; pfb: pectoral fin bud. D: Top view with ventral side to the left. E: Frontal view. F: lateral view. G and H: Embryos are in dorsal view. I and J: Embryos are in lateral view. |
|
Morphological defects of prep1.2 morphant embryos. A: Schematic design of antisense morpholinos and prep1.2-GFP construct. The morpholinos are complementary to 25 bp in the 5′ region spanning the ATG (MO1-prep1.2) or to 25 bp in the 5′UTR region (MO2-prep1.2); MO2-prep1.2 is non-overlapping with the 5′UTR of the prep1.2-GFP mRNA injected and can only block endogenous prep1.2 mRNA. Two control morpholino oligos were designed. They are mutated in 5 (MO1-prep1.2-5MIS) or 6 (MO2-prep1.2-6MIS) positions. B, C: Each prep1.2 morpholino was co-injected with prep1.2-GFP mRNA to test its specificity; only MO1-prep1.2 blocks prep1.2-GFP translation in injected embryos (compare C with control morpholino in B) as revealed by the GFP signal in cell nuclei of the head region. D, E; day 5 larvae injected with MO1-prep1.2 display morphological defects: lack of branchial tissue, impaired jaw organization, absence of pectoral fins and pigmentation defects. F?I; Alcian blue staining of 5-day-old larvae shows that Mekel′s cartilage is normal while hyoid cartilage was misshaped and reduced in size. Morphant embryos also display a reduction of the third pharyngeal cartilage while the more posterior ones are completely absent. ba: branchial arches; pf: pectoral fin; cb: ceratobranchial; ch: ceratohyal; e: ethmoid plate; me: Meckel′s cartilage; tr: trabeculae cranii. D and E: Embryos are in dorsal view. F and G: Embryos are in ventral view. H and I: embryos are in lateral view. PHENOTYPE:
|
|
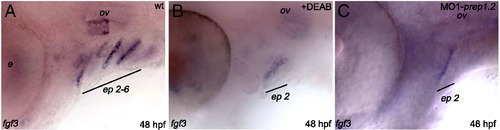
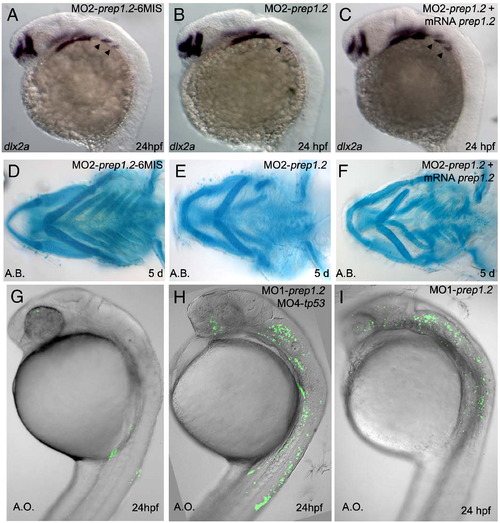
Characterization of prep1.2 function. A?J: Expression pattern of pre-migratory and migratory NCC markers. A, B: prep1.2 knock-down does not affect foxd3 expression in 10 somite stage embryos (compare A and B); C?F: dlx2a expression in NCC is unaffected in prep1.2 morphants analysed at 10 and 20 somite stages; G and H: at 24 hpf expression of dlx2a is strongly downregulated in the third stream of cephalic NCC of MO1-prep1.2 injected embryos; I and J: similar results are obtained in 48 hpf embryos: in prep1.2 morphant embryos dlx2a expression disappears in posterior branchial arches (J). K?P: Failure of the posterior pharyngeal endoderm segmentation in prep1.2 morphant embryos. K?N: Analysis of pharyngeal pouches development in the glu:GFP transgenic line that express GFP ectopically in the pharyngeal endoderm and in embryos stained with Zn5 antibodies. L: in MO1-prep1.2 injected embryos analyzed at 36 hpf, the more posterior pouches (ep3?ep5) do not develop (compare with control in K). Identical results were obtained when using Zn5 antibodies to stain for the pharyngeal endoderm (compare N with control in M, see also Supplemental Fig. 5). O and P; lack of fgf3 expression in the pharyngeal endoderm of prep1.2 morphants (F). A?F: dorsal view; G?P lateral view. b: branchial arches; ep: endodermal pouches; h: hyoid cartilage; m: Meckel′s cartilage; ov: otic vesicle. EXPRESSION / LABELING:
|
|
prep1.2 expression is positively regulated by retinoic acid. A, C and E: In situ hybridization experiments showing that prep1.2 mRNA in able to respond to RA treatment. In RA-treated embryos analyzed at 24 hpf the prep1.2 mRNA signal (blue, highlighted with a bar) is anteriorized and its intensity is significantly increased (C). In MO1-aldh1a2 injected embryos, prep1.2 expression is reduced, especially in the prospective branchial region (bar) (E). prep1.1 expression does not respond to RA (D) and is unaffected in aldh1a2 morphant embryos (F). G: qRT-PCR assay of prep1.2, hoxb1b and tbx1 expression in RA- and DEAB-treated embryos analyzed at 24 hpf. prep1.2 and hoxb1b are about 3-fold upregulated, while tbx1 is downregulated, in embryos treated with RA. DEAB treatment does not affect the expression of prep1.2 and tbx1 while hoxb1b is downregulated. The expression levels are compared with the housekeeping genes b-actin and eF1. H: Reporter analysis of the prep1.2 RARE. The basal promoter used in this study (p50-Luc) is unresponsive to RA. The 0.5 kbp intron-1 fragment bearing the putative prep1.2 RARE (p50-RARE-Luc) represses the basal promoter activity but confers a 7 fold RA-responsiveness. Mutagenesis of the RARE sequence of the 0.5 kbp intron-1 fragment restores the basal promoter activity. |
|
Pharyngeal endoderm analysis of DEAB-treated embryos and prep1.2 morphants. A?C: Zebrafish embryos are treated with DEAB from blastula stage up to 48 hpf. DEAB-treated embryos (B) and MO1-prep1.2 morphants (C) lack the posterior endodermal pouches. ep: endodermal pouches. |
|
aldh1a2 and hoxb1b expression is regulated by prep1.2. A?H: Starting from 15 somites to 24 hpf the expression of aldh1a2 in embryos injected with MO1-prep1.2 is reduced in branchial arches (black arrowhead), while aldh1a2 expression is unaffected in the trunk. In situ hybridization of 24 hpf embryos shows that aldh1a2 expression is lost also in pectoral fin bud (*) of prep1.2 morphant embryos. I?L: At 24 hpf hoxb1b expression is downregulated in branchial arches of prep1.2 morphant embryos. M, N: hoxb1b expression is unaffected at early developmental stages in MO1-prep1.2 injected embryos. Non-specific toxic effects of morpholino were excluded by co-injecting MO-p53 (H and L). ov: otic vesicle; pba: prospective branchial arches; pfb: prospective fin bud. |
|
Control experiments of Prep1.2 morpholinos. A: An embryo injected with a 6 bases mismatched prep1.2 morpholino develops two posterior streams of NCC (arrowheads) as evidenced by dlx2a expression pattern. B, C: loss of dlx2a expression in the posterior stream of NCC in embryos injected with MO2-prep1.2 is rescued when a prep1.2 mRNA lacking the morpholino target sequence is co-injected. D: An embryo injected with a 6 bases mismatched prep1.2 morpholino develops normal splanchnocranium. E, F: Rescue of defects in the posterior branchial arches of MO2-prep1.2 morphants by coinjection of a prep1.2 mRNA lacking the morpholino target sequence. G?I: Acridine orange staining in living embryos injected with prep1.2 morpholinos does reveal few p53-independent (G, n = 23) or p53-dependent (H, n = 27) cell death. |
|
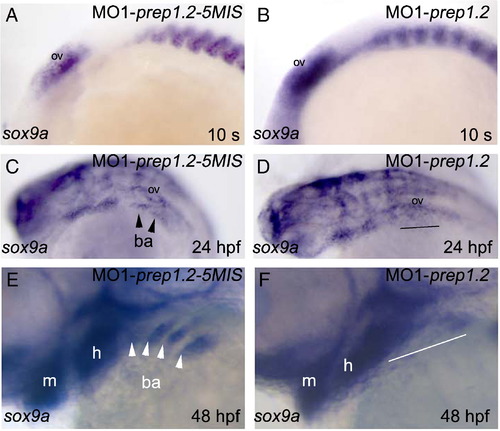
A?F: sox9a expression in cranial NCC of prep1.2 morphant embryos. A, B: sox9a expression in cranial NCC is unaffected in prep1.2 morphants analysed at the 10 somite stage. C?F: defective expression of sox9a in the branchial region of prep1.2 morphants analysed at 24 hpf (C and D) and 48 hpf (E and F). Lateral views, anterior to the left. |
|
Reduction of prep1.2 expression in RA defective embryos. A and B: In embryos treated with DEAB after NCC specification (form 15 somite stage), prep1.2 expression is reduced, in the mesenchyme of the branchial region (outlined). C and D: neckless mutants lacking prep1.2 expression in the prospective branchial region and in pectoral fin buds. |
|
Analysis of pharyngeal pouches development in embryos stained with Zn5 antibodies. B and D: in DEAB-treated embryos analyzed at 48 hpf, the more posterior pouches (p3?p5) do not develop (compare with control in A and C) (see also Figs. 3M and N). Lateral view, anterior to the left. C and D are higher magnifications of A and B, respectively. ep: endodermal pouches. |
|
RA and prep1.2 depletion does not affect the number of pharyngeal endodermal cells. A?C: her5 expression at tailbud stage is indistinguishable in controls (A), prep1.2 morphants (B) and DEAB-treated embryos (C). Embryos are in dorsal views with anterior to the top. |
|
prep1.1 and prep1.2 have non-overlapping functions in hindbrain and head mesenchyme, respectively. A?L: marker expression shows mild differences in the hindbrain between controls (left column) and embryos injected with MO1-prep1.2 (right column). C?D and I?J: expression of hoxb2 and hoxb3a is upregulated posterior to r5 and r7, respectively. E?F: reduction of mafb expression in the NCC of the third stream of prep1.2 morphants. M, N: similar aldh1a2 expression in the prospective branchial arches and pectoral fin buds of MO1-prep1.1 injected embryos and their control. All embryos are in dorsal views with anterior to the left. ov: otic vesicle; pba: prospective branchial arches; pfb: pectoral fin bud; r: rhombomere. |
Reprinted from Developmental Biology, 343(1-2), Vaccari, E., Deflorian, G., Bernardi, E., Pauls, S., Tiso, N., Bortolussi, M., and Argenton, F., prep1.2 and aldh1a2 participate to a positive loop required for branchial arches development in zebrafish, 94-103, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.