- Title
-
Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion
- Authors
- Duldulao, N.A., Lee, S., and Sun, Z.
- Source
- Full text @ Development
|
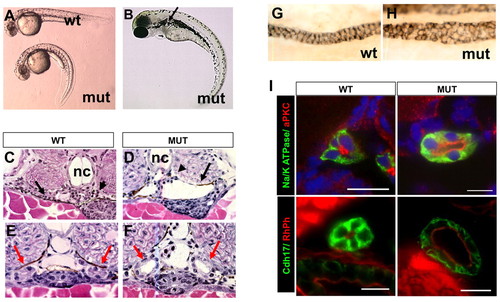
Phenotypes of scohi459 mutant zebrafish. (A) Side view of embryos at 36 hpf. (B) A mutant at 3 dpf. The arrow points to a cyst. (C,D) Cysts at the glomerular (arrowhead) and tubular (arrow) regions in cross-sections of mutant embryos at 50 hpf. (E-I) Increased size of the pronephric duct in mutant embryos as shown in cross-sections of the duct region (E,F red arrows) at 50 hpf. (G,H) The pronephric duct in side view in whole-mount embryos stained with α6F. (I) Cross sections of pronephric duct at 5 dpf. Anti-Na+/K+-ATPase and anti-Cdh17 are used as basolateral markers. Atypical PKC (aPKC) and rhodamine phalloidin (RhPh) are used as apical markers. DAPI is used as nuclei dye. mut, mutant; nc, notochord; wt, wild type. Scale bars: 10 μm. |
|
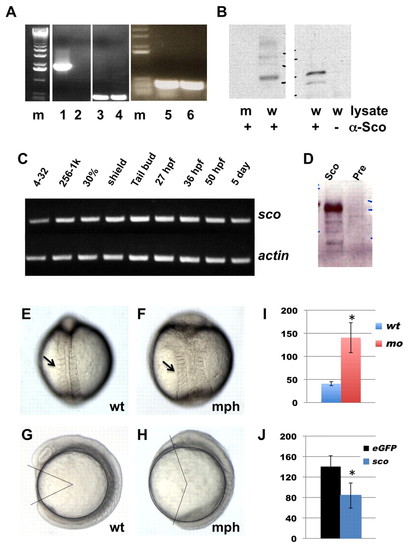
hi459 is a zygotic null allele of arl13b (sco). (A) arl13b transcripts in embryos at 5 dpf shown by RT-PCR of cDNA from hi459 mutant embryos (lanes 2, 4, 6) and wild-type siblings (lanes 1, 3, 5). m, size marker; lanes 1-2, RT-PCR with a pair of primers spanning the proviral insertion site in arl13b; lanes 3-4, RT-PCR with a pair of primers for beta-actin as loading controls; lanes 5-6, RT-PCR with a pair of primers 3′ to the proviral insertion site in arl13b. (B) Absence of Arl13b in hi459 mutant embryos at 5 dpf. A band of the predicted size can be seen in samples immunoprecipitated with anti-Sco from lysate of wild-type siblings (w), but not in the sample from hi459 mutant embryos (m), nor in the lysate precipitated with protein A beads alone. (C) RT-PCR time-course for arl13b expression. 4-32, four- to 32-cell stage; 256-1k, 256- to 1000-cell stage; 30%, 30% epiboly. (D) Presence of Arl13b in embryos at the 64-cell stage. Lysates from embryos at the 64-cell stage were subjected to immunoprecipitation using anti-Sco (Sco) or pre-immune IgG (Pre) and precipitated proteins detected by western analysis using anti-Sco. (E-H) Wild type (wt) and arl13b morphant (mph) embryos in dorsal view (E,F) and side view (G,H) at around the 8-somite stage. Arrows in E and F point to somites. In G and H, body gap angle was labeled using ImageJ. (I) Gap angle in degrees in wild type (wt, n=13) embryos and arl13b morphants (mo, n=14; *P<0.001). (J) Gap angle in degrees in arl13b morphants co-injected with eGFP (eGFP, n=13) or sco mRNA (sco, n=15; *P<0.001). PHENOTYPE:
|
|
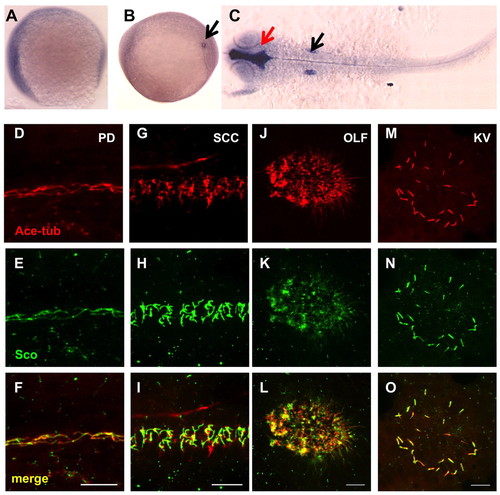
Gene products of arl13b are widely distributed. (A-C) In situ hybridization for arl13b on embryos at the 1-somite stage (A), the 8-somite stage (B) and 25 hpf (C). A and B are side views and C is a dorsal view, all with anterior to the left. In B, the transcript is enriched in the Kupffer′s vesicle (KV, arrow). In C, enrichment in the brain ventricle (red arrow) and the otic vesicle (black arrow) can be seen. (D-O) Arl13b is highly enriched in cilia in multiple organs as shown by whole-mount immunostaining of embryos at 2 dpf (D-L) and the 10-somite stage (M-O). The cilia marker anti-acetylated tubulin is shown in red, while anti-Sco is shown in green. (D-F) The pronephric duct (PD). (G-I) The spinal cord canal (SCC). (J-L) The olfactory placode (OLF). (M-O) The KV. Scale bars: 10 μm. EXPRESSION / LABELING:
|
|
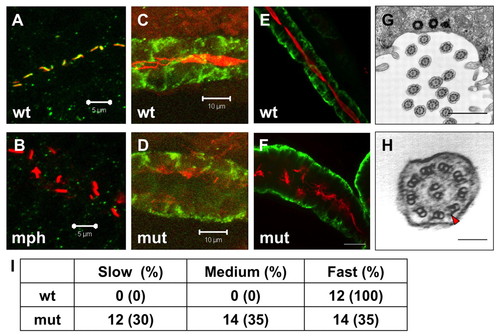
Cilia in scohi459 mutant embryos. (A,B) Confocal projections showing the pronephric duct in a wild-type embryo (A) and an arl13b morphant (B) at the 20-somite stage, stained with anti-acetylated tubulin (red) and anti-Sco (green). Signal in B is overexposed. Scale bars: 5 μm. (C,D) Confocal projections showing cilia defect (stained with anti-acetylated tubulin, red) in the pronephric duct (stained with anti-Cdh17, green) in a scohi459 mutant embryo (D), compared with a wild-type sibling (C), at 33 hpf. Scale bars: 10 μm. (E,F) Confocal projection showing cilia (stained with anti-acetylated tubulin, red) in the pronephric duct (stained with anti-Cdh17, green) in embryos at 5 dpf. Scale bar: 20 μm. (G,H) Electron micrographs showing the ultrastructure of cilia in the scohi459 mutant at 5 dpf. H shows an enlarged cross section; red arrowhead indicates a dynein arm. Scale bars: 1 μm in G; 100 nm in H. (I) Cilia motility in scohi459 mutant and wild-type siblings at 5 dpf. Cilia with a beating frequency of between 36 and 55 Hz are categorized as fast, whereas cilia with frequency of between 21 and 35, and between 5 and 20 are categorized as medium and slow, respectively. mph, morphant; mut, mutant; wt, wild type. EXPRESSION / LABELING:
|
|
arl13b is required for proper left-right asymmetry. (A) In situ hybridization for cardiac myosin light chain (cmlc2) showing the heart tubes of wild-type and morphant embryos at 26 hpf. (B) Tabulation of heart position from three independent experiments. (C) In situ hybridization for southpaw on wild-type and morphant embryos at the 18- to 20-somite stage, in dorsal view with head to the top. (D,E) KV in a wild-type embryo (D) and a morphant (E). Scale bars: 20 μm. Ab, absent; Bi, bilateral; L, left-sided; M, middle; mph, morphant; R, right-sided; wt, wild type. EXPRESSION / LABELING:
PHENOTYPE:
|
|
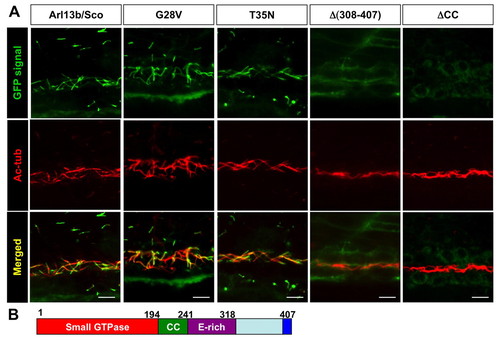
Multiple regions, but not the small GTPase activity, of Arl13b are required for its targeting to the cilium. (A) Images show side views of the pronephric duct in embryos injected with mRNA encoding eGFP-tagged versions of Arl13b indicated above. eGFP signal is shown in green, while anti-acetylated tubulin (Ac-tub) is labeled in red. CC, coiled-coil domain. Scale bars: 10 μm. (B) Schematic of the structure of Arl13b. Numbers are amino acid coordinates. |
|
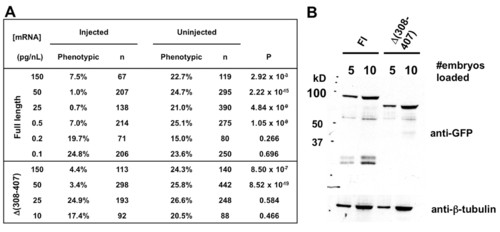
Localization and functions of Δ(308-407) Arl13b in vivo. (A) arl13b::gfp mRNA retains detectable rescue activity at a quantity 100-fold less than that of Δ(308-407)::gfp mRNA. (B) Arl13b::GFP and Δ(308-407)::GFP are expressed at similar levels when the same amount of mRNA is injected. |
|
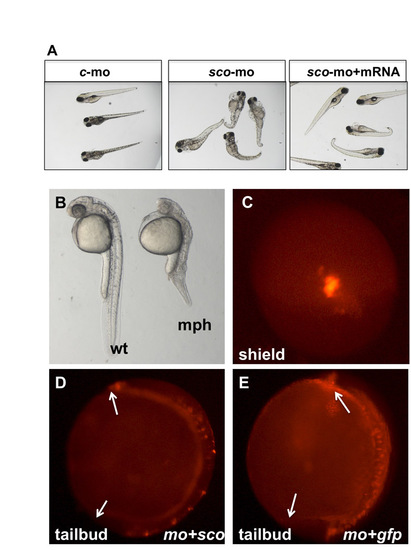
Phenotypes of arl13b morphants. (A) Injected at modest dosage, arl13 morphants show similar phenotypes to scohi459 mutants. The morphants can be rescued by co-injection of arl13b mRNA. c-mo, embryos injected with a standard control morpholino; sco-mo, embryos injected with the arl13b morpholino; sco-mo+mRNA, embryos co-injected with arl13b morpholino and mRNA. (B) Injected at higher dosage, arl13 morphants show more severe phenotypes at 1 dpf. mph, morphant; wt, wild type. (C) A shield-stage embryo injected with FM1-43 in dorsal view. (D,E) Side views of injected embryos at the tailbud stage. Arrows point to the tip of the head and the tail. mo+sco, embryos co-injected with sco morpholino and mRNA (D); mo+gfp, embryos co-injected with sco morpholino and eGFP mRNA (E). |
|
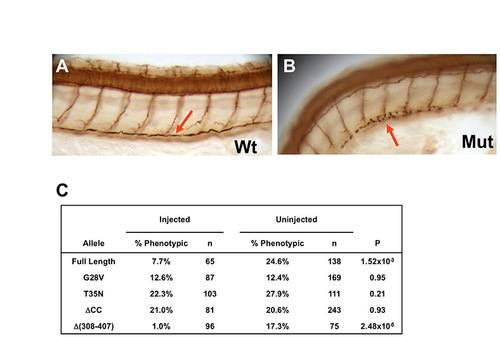
Point mutations in the small GTPase domain, and coiled-coil deletion disrupt cilia rescue activity. (A,B) Cilia in the pronephric duct (arrow) at 50 hpf as shown by anti-acetylated tubulin immunostaining in whole-mount embryos; side views at low magnification. Mut, scohi459 mutant sibling; wt, wild type. (C) 265.5 pg mRNA encoding alr13b alleles translationally fused to gfp were injected into hi459+/-xhi459+/- embryos at the one- to four-cell stage. Embryos were then scored at 2 dpf for body curvature, and stained with antibody against acetylated tubulin to evaluate the pronephric cilia phenotype. P-value is calculated from a chi-squared test with 1 degree of freedom. Results from a representative experiment are shown. |