- Title
-
Position fine-tuning of caudal primary motoneurons in the zebrafish spinal cord
- Authors
- Sato-Maeda, M., Obinata, M., and Shoji, W.
- Source
- Full text @ Development
|
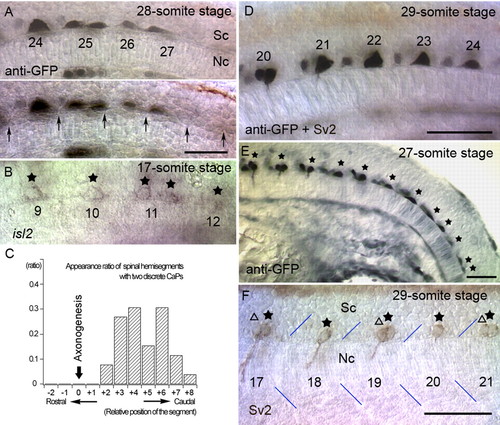
The pattern of CaP cell bodies before and after axonogenesis. (A) Optical sections at different focal levels of an nrp1a:gfp transgenic embryo at the 28-somite stage (23 hpf). CaPs are labeled with anti-GFP antibody. Position of CaP cell bodies was not related to the corresponding somites. Also, two distinct and discrete cell bodies were observed at the 25th somite level. Arrows (lower panel) indicate the somite boundaries. (B) Irregular patterns of CaP cell bodies were also observed as the pattern of isl2-expressing cells (stars) during the pre-axonogenesis period in wild-type embryos. Two separate CaP cells were observed at the 11th somite level. (C) Appearance ratio of spinal hemisegments with two discrete CaPs. The two discrete CaPs were only seen in caudal segments where CaPs did not begin axonogenesis. Position 0 is defined as the most caudal segment in which a CaP axon was formed. Thirty-three pairs of separate CaPs from 26 embryos at the 26- to 29-somite stages were sorted by relative somite levels and scored for spinal hemisegments with two separated CaPs. (D) A transgenic embryo at the 29-somite stage (23.5 hpf). Although CaP cell bodies with axons were ellipsoid (20th, 21st), more caudal CaP cells appeared triangular or trapezoid (23rd, 24th). (E) Side view of a transgenic embryo at the 27-somite stage (22.5 hpf). (F) CaP cell bodies with axons were regularly spaced in the middle of overlying somites. Stars and triangles show mAb Sv2-labeled CaP/VaP cell bodies. Blue lines denote somite borders. Unless noted otherwise, embryos are oriented as rostral to the left and dorsal up. Sc, spinal cord; Nc, notochord. The numbers in the panels indicate the segmental order. Scale bars: 50 mm. |
|
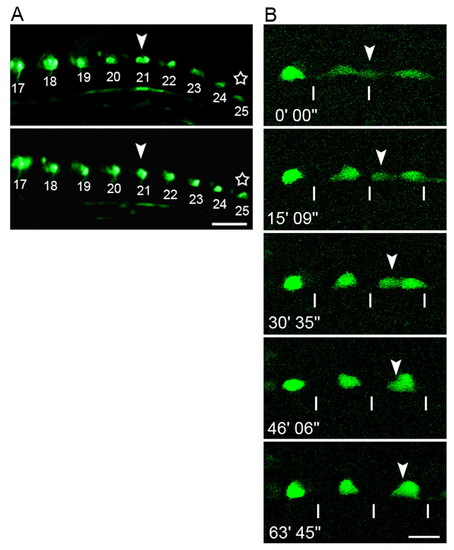
Live observations of CaP cell bodies in nrp1a:gfp transgenic embryos. (A) Trunk region of the same embryo shown at different stages. Two discrete cells underlying a single somite seen in the upper panel (the 21st somite level at the 27-somite stage: arrowhead) overlapped after 80 minutes, shown in the lower panel. A transition in cell shape between different stages was also observed (stars). The numbers in the panels indicate the segmental order. Scale bar: 50 Ám. (B) Confocal micrographs of CaP cell bodies in another embryo. Sequential images were captured of the 16-18th segment, starting from the 17-somite stage (17.5 hpf). A CaP cell body originally located beneath the somite boundary (arrowhead) migrated posteriorly and overlapped with another CaP in the middle of the somite. The numbers denote the time elapsed. The lines indicate segment borders of the somites. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
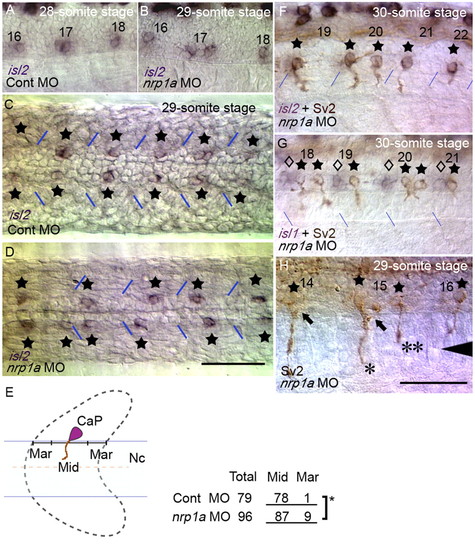
Knockdown of Nrp1a results in abnormal CaP cell positioning even after axon formation. Position of CaP cell bodies after axonogenesis was examined using isl2 expression (purple) and mAb Sv2 (brown). (A) Side view of a control embryo at the 28-somite stage. (B) A Nrp1a-knockdown embryo at the 29-somite stage with an irregular CaP pattern. (C,D) Horizontal sections of control and Nrp1a-knockdown embryos. CaPs are indicated by stars. (E; left) Severe dislocation was defined as the center of the CaP cell bodies being located in either the anterior or the posterior quarter of the overlying somite (indicated by `Mar' in the schema). (Right) Dislocation of CaP was significantly increased in Nrp1a-knockdown embryos, as determined by Fisher's exact test of independence (star, P<0.05).Nc, notochord. (F) Abnormal exit points of CaP axons by two separate CaPs in Nrp1a-knockdown embryos. CaP axons were labeled with mAb Sv2. (G) The positions of CaPs (stars) relative to MiP and RoP (diamonds, labeled by isl1) were unaffected by Nrp1a knockdown. (H) Different lengths of two axons from separate CaPs correspond to those of a CaP and a VaP. One axon extended onto the ventral myotome (*), whereas the other axon did not extend beyond the horizontal myoseptum (**). Arrowhead shows the level of the horizontal myoseptum. MiP axons extended normally along the dorsal pathway (arrows). The numbers in A, B and F-H denote the somitic segment order. The blue lines indicate segment borders. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
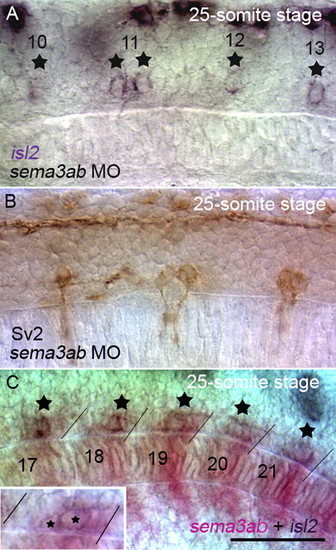
Knockdown of Sema3ab causes the double exit phenotype of CaP axons similar to Nrp1a knockdown. (A) The position of CaP cell bodies after axonogenesis was examined using isl2 expression. Separate isl2+ CaP cell bodies were detected in Sema3ab-knockdown embryos (11th segment of a 25-somite stage embryo). (B) The double exit phenotype of CaP axons was observed in a Sema3ab-knockdown embryo. CaP axons and cell bodies were labeled with mAb Sv2. (C) sema3ab was expressed in the posterior halves of somites, and CaP cell bodies (detected by isl2 expression) were observed around the margin of the sema3ab expressing region. The inset shows the 21st segment of the same embryo at the focal plane of the CaP cell bodies. In older segments in which CaP axonogenesis had begun, expression of sema3ab was downregulated (17th segment of a 25-somite stage embryo). The numbers indicate the segmental order, and the stars indicate the positions of the CaP cell bodies. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
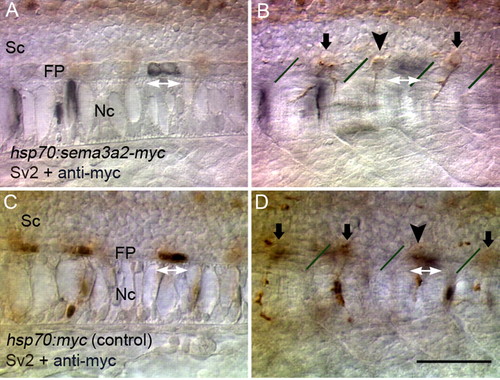
Abnormal CaP cell positioning induced by focal ectopic Sema3ab. (A,B) Optical sections at different focal levels of an embryo in which Sema3ab-Myc was transiently expressed. Ectopic Sema3ab was labeled with anti-Myc (black) and CaPs with mAb Sv2 (brown). In a case in which ectopic Sema3ab was expressed in floor plate cells (white double arrows in A), the CaP cell body was shifted anteriorly (arrowhead in B) away from the cells expressing Sema3ab. (C,D) Optical sections of a control embryo. Myc epitope expression (white double arrows in C) did not affect the location of the CaP cell body (arrowhead in D) and overlapped with it. Lines in B and D indicate somitic borders. Sc, spinal cord; FP, floor plate; Nc, notochord. Scale bar: 50 μm. |
|
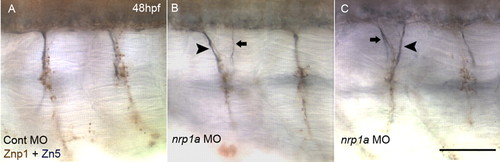
Aberrant exit points of secondary motoneuron (SMN) axons in Nrp1a-knockdown embryos. (A) Axons of primary motoneurons (PMNs) and SMNs at 48 hpf in a control MO-injected embryo. PMN axons were labeled with a brown stain, recognized by mAb Znp1, but not by Zn5 (Zeller et al., 2002). SMN axons were stained in blue-black, recognized by Zn5 (Fashena and Westerfield, 1999), regardless of Znp1 reactivity. SMN axons extended ventrally along with PMN axons using an exit point common to PMN and SMN. (B) Double exit phenotype of SMN axons was observed in Nrp1a-knockdown embryos at 48 hpf. Generally, one axonal bundle of SMNs was thicker (arrowhead) than the others (arrow), regardless of its anterior or posterior position. (C) Another example of a double exit phenotype of SMN. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
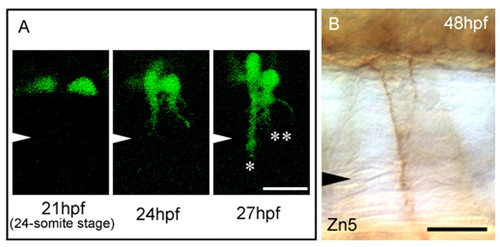
Abnormal CaP cell positioning results in double exit points in both primary motoneurons (PMNs) and secondary motoneurons (SMNs). (A) Sequential images at the 18th somite level in an nrp1a:gfp transgenic embryo with Nrp1a-knockdown. Two separated CaP cell bodies (21 hpf) extended their axons to form double exit points (24 hpf). At 27 hpf, one axon extended beyond the horizontal myoseptal level (*), but the other axon did not (**). Arrowheads indicate the level of the horizontal myoseptum. (B) The double exit phenotype of SMN axons was observed at the same position as in A at 48 hpf. SMN axons were immunostained with mAb Zn5. Scale bars: 20 μm. EXPRESSION / LABELING:
|