- Title
-
Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia
- Authors
- Blechman, J., Borodovsky, N., Eisenberg, M., Nabel-Rosen, H., Grimm, J., and Levkowitz, G.
- Source
- Full text @ Development
|
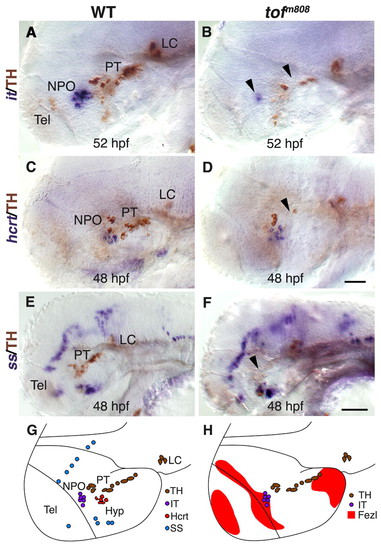
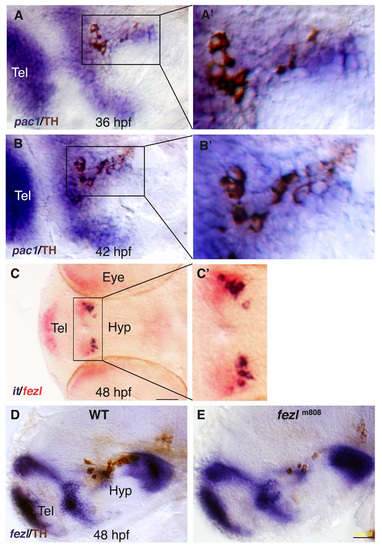
Analysis of hypothalamic neurons in fezl/tofm808 mutant embryos. (A-F) Heterozygous (WT; A,C,E) embryos and their too fewm808 (tofm808) mutant siblings (B,D,F) were fixed at 52 (A,B), and 48 (C-F) hours post fertilization (hpf) and subjected to whole-mount in situ hybridization with antisense RNA probes directed against either the hypothalamic neuropeptide isotocin, which is the zebrafish ortholog of oxytocin (it; A,B), hypocretin/orexin (hcrt; C,D) or somatostatin (ss; E,F). After probe color development, all specimens were subjected to immunostaining with an anti-tyrosine hydroxylase (TH) antibody to detect DA neurons. WT heterozygous and tofm808 embryos were scored by TH staining followed by sequencing-based genotyping. Black arrowheads indicate deficiencies in DA and IT neurons in too few embryos. (G,H) SchCsup>m808 embryos were scored by TH staining followed by sequencing-based genotyping. Black arrowheads indicate deficiencies in DAematic representations of the examined hypothalamic cell types and of fezl/tof expression domains in a 2-day-old WT embryo. All panels show lateral views of the embryo, anterior to the left. Hyp, hypothalamus; LC, locus coeruleus; NPO, neurosecretory preoptic area; PT, posterior tuberculum; Tel, telencephalon. Scale bars: 50 μm in A-D; 100 μm in E,F. |
|
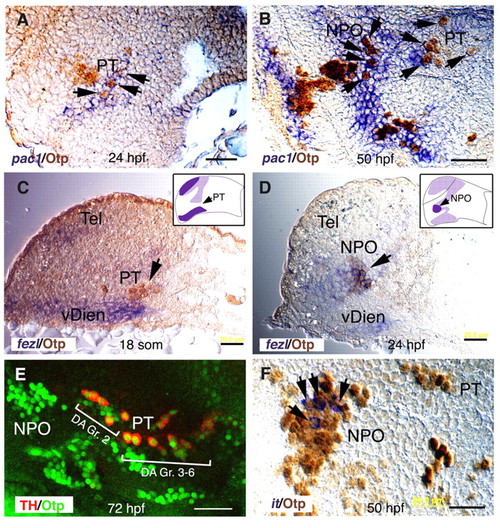
Coordinated expression of pac1, fezl and Otp in the NPO and PT areas. (A-D,F) Sagittal paraffin sections (6 μm) through the NPO and PT (lateral view, anterior to the left). At the indicated stages of development, embryos were subjected to whole-mount in situ hybridization with probes directed against pac1 (A,B), fezl (C,D) or isotocin (it; F). Thereafter, specimens were sectioned and immunostained with an anti-Otp antibody. Schemes of fezl expression domain (dark purple) at the plane of the section are shown in C and D. Labeled RNA probes were detected in the cytoplasm, whereas Otp antibody exclusively labeled the nuclei. (E) Projected confocal Z-stack images of embryos (lateral view, anterior to the left) that were subjected to double immunofluorescence staining with monoclonal and polyclonal antibodies directed against tyrosine hydroxylase (TH) and Otp, respectively. The two prominent clusters of dopaminergic neurons, group 2 (Gr. 2) and groups 3-6 (Gr. 3-6), are indicated. hpf, hours post fertilization; NPO, neurosecretory preoptic area; PT, posterior tuberculum; vDien, ventral diencephalon. Scale bars: 20 μm in A-D; 40 μm in E,F. EXPRESSION / LABELING:
|
|
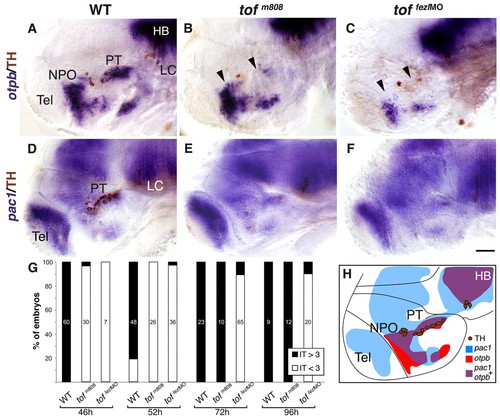
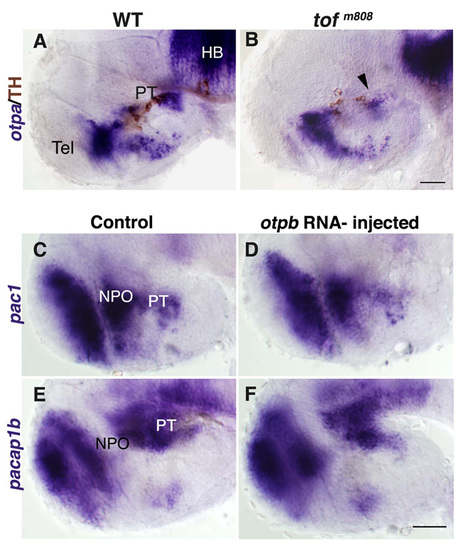
Aberrant otpb expression in fezl-deficient embryos. Embryos (at 52 hpf, lateral view) were subjected to whole mount in situ hybridization with either antisense otpb (A-C) or pac1 (D-F) RNA probes followed by immunostaining with an antibody directed against tyrosine hydroxylase (TH; A-F) that detects DA neurons. (A,D) Wild type (WT). (B,E) tofm808 mutants. (C,F) tofm808 embryos were injected, at one-cell stage, with an antisense Fezl morpholino (fezlMO) that blocks proper splicing leading to retention of intron 2 and a shorter protein that lacks most of the zinc finger domain (sp3; see Fig. S6 in the supplementary material). WT heterozygous and tofm808 embryos were scored by TH staining followed by sequencing. Black arrowheads mark deficient otpb expression domains. (G) Delayed IT development in tofm808. Relative number of IT/OT neurons in too few (tofm808) embryos, toffezlMO and their WT siblings at different developmental stages. toffezlMO indicate embryos, in which fezl-directed antisense morpholino was injected into tofm808. Embryos were scored by co-staining of IT and TH followed by sequencing of the tofm808 mutation. WT embryos display 4-7 IT cells (IT>3) on each side of the brain whereas tofm808 display a deficit (IT<3) in IT cell number between 46 and 52 hours of development. The bars were normalized to 100%. The number of scored embryos is indicated on each bar. (H) A scheme depicting an overlay of TH, otpb and pac1 expression domains as they appear in WT embryos (at 52 hpf). HB, hindbrain; LC, locus coeruleus; NPO, neurosecretory preoptic area; PT, posterior tuberculum; Tel, telencephalon. Scale bar: 50 μm. |
|
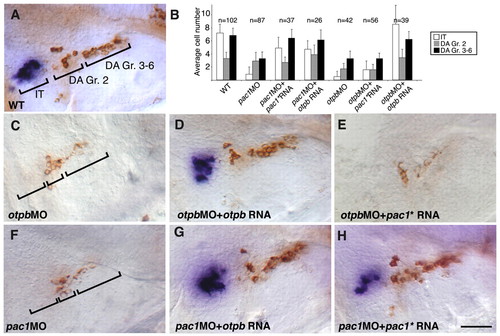
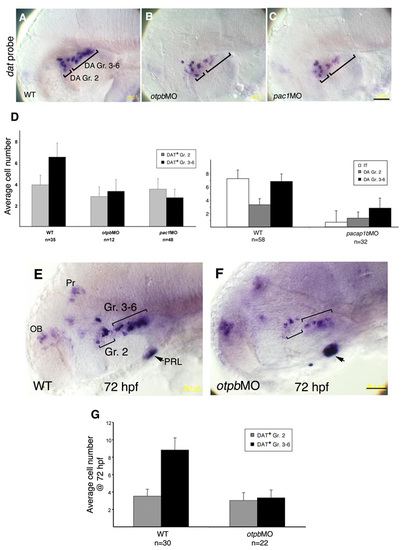
Genetic interactions between pac1 and otpb. (A,C-H) High-resolution micrographs of embryos (at 52 hpf, lateral view, anterior to the left) that were subjected to in situ hybridization with an isotocin (IT)-directed probe, followed by immunostaining with an anti-tyrosine hydroxylase (TH) antibody. The two prominent clusters of dopaminergic (DA) neurons (Gr. 2 and Gr. 3-6), stained in brown, as well as isotocinergic (IT) neurons, stained in purple, are indicated. (B) Bar chart showing average cell counts of isotocinergic (IT), dopaminergic Group 2 (DA Gr. 2) and Groups 3-6 (DA Gr. 3-6). Error bars indicate s.d. The number of embryos (n) is shown above. In order to represent unilateral cell number (as shown in the micrographs), neurons were counted on both sides of the brain and the total number was divided by two. Statistical analysis for all treatments was done by ANOVA test. Wild-type (WT) embryos were injected with antisense morpholinos directed against either otpb (otpbMO; C,D,E and see Fig. S7 in the supplementary material) or pac1 (pac1MO; F,G,H). (C-H) Embryos were injected with in vitro synthesized capped mRNA encoding either Otpb (D,G; at 7-15 pg per embryo) or a mutant form of PAC1 (denoted pac1*) containing a single E239Q base substitution that renders it constitutively active (E,H; at 200pg per embryo). Injected pac1* and otpb RNAs were constructed without their respective morpholino-binding site, thus enabling a genuine gene-function complementation rather than competition for morpholino binding. The amount of injected mRNA in Otpb and PAC1 gain-of-function experiments was determined by titrating the minimal doses of mRNA that could rescue otpb and pac1 morphant phenotype, respectively. Embryos that showed abnormal brain morphology were omitted from our analysis. Scale bar: 50 μm. |
|
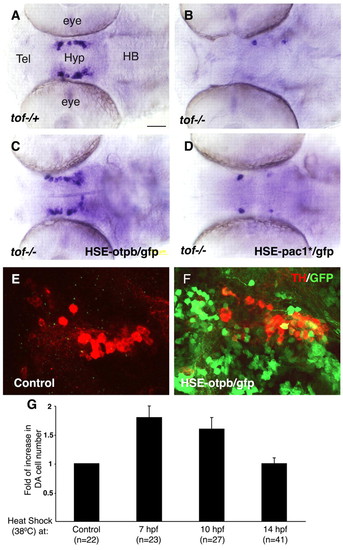
Otpb is a critical downstream effector of Fezl in DA progenitors. (A-F) Embryos were injected with either a buffer solution (A,B,E) or DNA constructs containing a heat shock element (HSE) expression cassette that drives a GFP tracer together with either otpb (C,F; at 10-20 pg per embryo) or the constitutively active pac1* (D) cDNAs and were grown at 28°C. At 7 hours post fertilization (hpf) embryos were transferred to a permissive temperature of 38°C for a period of 45 minutes and were shifted back to a 28°C incubator. Mosaic embryos expressing the GFP tracer in the ventral diencephalon were selected for further analysis and were thereafter harvested at 52 hpf. DA neurons were scored by either an RNA probe directed to dopamine transporter (dat; A-D) or an anti-tyrosine hydroxylase (TH) antibody (E,F). A-D are siblings derived from a cross between tofm808-/- and tofm808-/+ fish. Subsequent to the phenotypic analysis, embryos were separated into a multiwell dish and were genotyped by sequencing. (G) Bar chart showing the relative changes in DA cell number following conditional activation of Otpb in WT embryos that were injected with a plasmid harboring a heat shock element-driven otpb/gfp (HSE-otpb/gfp) expression cassette. At the indicated times after fertilization, embryos were subjected to a 45 minute temperature shock (38°C) and the number of dat+ hypothalamic DA neurons in each embryo was scored at 52 hpf. Scale bar: 50 μm. |
|
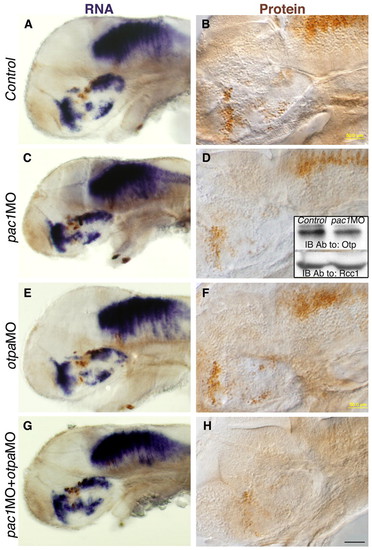
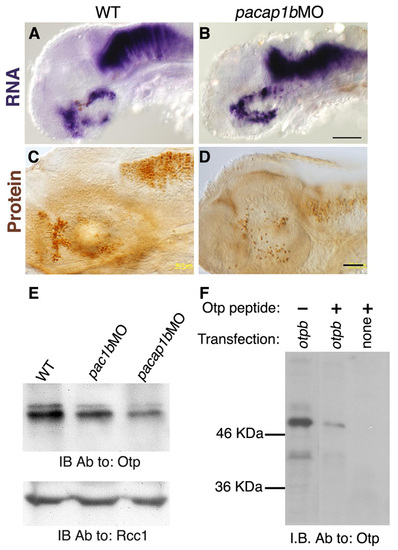
Regulation of the Otp protein by PACAP/PAC1 signaling pathway. (A-H) Embryos were injected with either a control pac1 missense morpholino (Control, A,B), or with antisense morpholinos directed against pac1 (pac1MO, C,D), otpa (otpaMO, E,F) or combination of pac1+otpa (pac1MO+otpaMO, G,H) and were thereafter (52 hpf) subjected to either in situ hybridization with an otpb-directed probe (A,C,E,G), or to immunohistochemistry with an anti-Otp antibody (B,D,F,H). Inset in panel D shows western blot analysis of nuclear-enriched protein extracts from control and pac1MO-injected embryos using antibodies directed to either Otp or the nuclear protein Rcc1 (internal protein loading control). The activity of each morpholino was evaluated by anti-tyrosine hydroxylase (TH) immunostaining. Scale bars: 100 μm in A,C,E,F; 50 μm in B,D,F,H. EXPRESSION / LABELING:
|
|
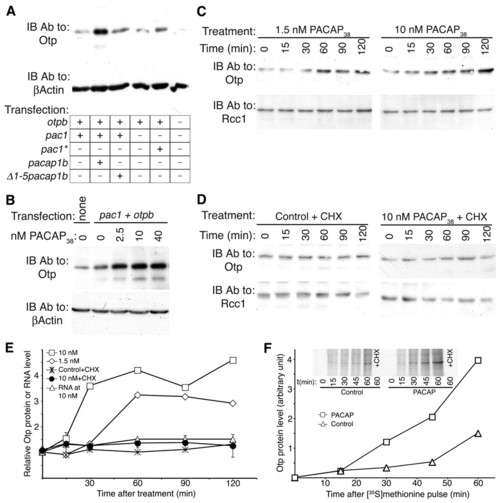
PACAP regulates Otp post-transcriptionally. (A,B) Western blot of equal amounts of protein extracts from HEK293 cells with an anti-Otp antibody followed by an anti-β-actin antibody that served as an internal reference. (A) HEK293 cells were transiently transfected with different combinations of zebrafish cDNAs as indicated and harvested 24 hours post transfection. pac1* encoded a constitutively activated form of pac1. The full-length form of zebrafish pacap1b precursor gene, gives rise to several proteolytically cleaved neuropeptides including growth hormone releasing hormone (GHRH) and PACAP1b (Wang et al., 2003). Δ1-5pacap1b cDNA had an internal deletion of the first five amino acids of the mature PACAP peptide. (B) HEK293 cells were transfected with the indicated zebrafish cDNAs and 24 hours later monolayer cultures were treated with different concentration of a synthetic PACAP38 peptide for a period of 120 minutes. (C,D) Western blot of nuclear-enriched protein extracts from PC12 cells using either an anti-Otp or anti-Rcc1 antibody (internal control for a ubiquitous nuclear protein). Cells were treated with different concentrations of either PACAP38 peptide alone (C), 50 μg/ml cycloheximide (Control+CHX) or PACAP38+CHX (D) and harvested at the indicated time points. (E) Standardized Otp protein and RNA (n=6) levels as a function of time after PACAP38 application to PC12 cells. otp RNA levels were determined by quantitative real-time PCR (RNA at 10 nM). (F) Kinetics of newly synthesized Otp protein in the absence (Control) or presence of 10nM PACAP. PC12 cells were incubated with methionine-free medium containing [35S]methionine (at 100 μCi/ml). Protein extracts were made at the indicated times and equal amounts of total protein were subjected to immunoprecipitation with an anti-Otp antibody followed by gel electrophoresis and autoradiography (inset). The correct position of the Otp band was confirmed by performing immunoprecipitation and western blot on the same extracts and by Otp peptide displacement. Ab, antibody; CHX, cycloheximide; IB, immunoblot. |
|
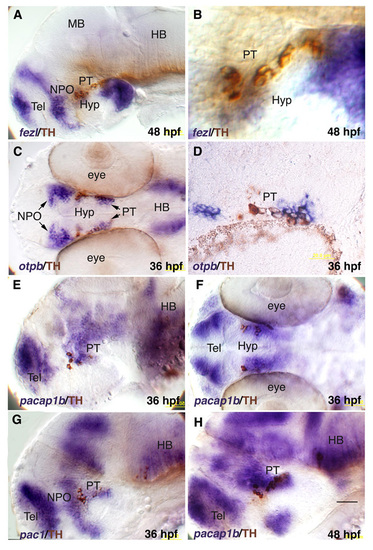
Expression of hypothalamic genes relative to DA neurons. In situ hybrydization with RNA probes directed against fezl (a,b), otpb (c), pacab1 (e,f,h) and pac1 (g) followed by immunostaining with an anti-tyrosine hydroxylase (TH) antibody. (D) Sagittal paraffin sections (6 μM) of an embryo which was subjected to in situ hybridization with an otpb- directed probe followed by anti-TH immunohistochemistry. HB, hindbrain; hpf, hours post fertilization; Hyp, hypothalamus; MB, midbrain; NPO, neurosecretory preoptic area; PT, posterior tuberculum; Tel, telencephalon. |
|
In situ hybridization with pac1, isotocin (it) and fezl RNA probes. (A,B) High resolution micrographs (lateral view) of embryos which were subjected to in situ hybridization with RNA probes directed against pac1 followed by anti-TH immunohistochemistry. (C) Double in situ hybridization with RNA probes directed against isotocin (it) and fezl (ventral view). (D,E) Wild type (WT; D) and tofm808 (E) mutant with antisense fezl probe followed by anti-TH immunohistochemistry. |
|
Antisense morpholinos to otpb, pac1 and pacap1b affect dopamine transporter (DAT)-positive DA neurons. (a-c,E,F) Embryos were injected with either a buffer solution (WT; A,E), otpb morpholino (otpbMO; B,F) or pac1 morpholino (pac1MO; c) and were thereafter subjected to in situ hybridization with either a dopamine transporter (dat)-directed probe (A-C; at 52 hpf) or dat+prolactin (PRL)-directed probes (E,F; at 72 hpf). The two prominent clusters of dopaminergic (DA) neurons (Gr. 2 and Gr. 3-6), are indicated. (d,G) Histograms showing the effects of otpb, pac1 and pacap1b morpholinos on average cell counts of DAT+ dopaminergic (DA) and isotocinergic (IT) neurons. Bars indicate the standard deviation. The number of embryos (n) is shown below. To represent unilateral cell number (as shown in the micrographs), neurons were counted on both sides of the brain and the total number was divided by two. Scale bar: 50 μm. |
|
PACAP1b affects Otp protein levels. (a-D) Embryos were injected with either a buffer solution (WT, a,C) or pacap1b morpholino (pacap1bMO; B,D) and were thereafter (52 hours post injection) subjected to either in situ hybridization with an otpb-directed probe (a,B), or to immunohistochemistry with an anti-Otp antibody (C, D). The activity of pacap1bMO in each embryo was then evaluated by anti-tyrosine hydroxylase (TH) immunostaining. (E) Nuclear-enriched protein extracts from mock (WT), pac1MO and pacap1bMO injected embryos were subjected to western blot analysis with either anti-Otp antibody or anti-Rcc1 (internal control for a ubiquitous nuclear protein). (F) Specificity of the anti-Otp antibody. HEK293 cells were transfected with a cDNA encoding to the Otpb protein and monolayers were cultured for 24 hours before protein extraction and western blot. Specific immunoreactivity against a major protein band of ∼50 kDa was demonstrated by peptide antibody displacement. PVDF filters containing the fractionated cell lysate were then subjected to immunobloting (IB) with an affinity-purified anti-Otp antibody, which was either pre-incubated (+) with a synthetic Otp peptide or not (-). Ab, antibody; IB, immunoblot. Scale bars: (a,B) 100 μm; (C,D) 50 μm. EXPRESSION / LABELING:
|
|
Effects of otpa antisense RNA probes and otpb mRNA. Wild-type (WT; a) embryos and their too fewm808 (tofm808) mutant siblings (B) were fixed at 52 hours post fertilization and subjected to whole-mount in situ hybridization with antisense RNA probes directed against otpa followed by immunostaining with an antibody directed against tyrosine hydroxylase (TH). Black arrow heads mark deficient otpa expression domains. (C-F) Embryos were injected with either a buffer solution (Control, C,E) or otpb mRNA (E,F) and were thereafter (48 hours post injection) subjected to either in situ hybridization with probes directed to either pac1 (C,D) or pacap1b (E,F). HB, hindbrain; PT, posterior tuberculum; Tel, telencephalon. Scale bars, 50 μm. EXPRESSION / LABELING:
|
|
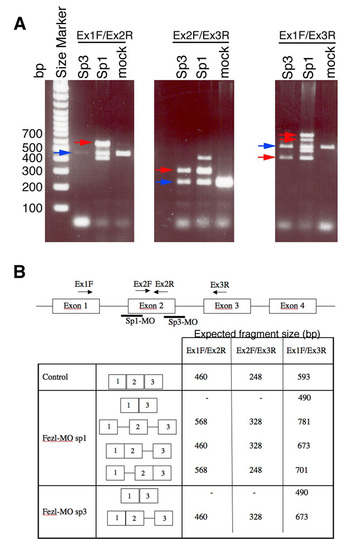
Effects of fezl-directed splice-blocking morpholinos. (a) Gel electrophoresis image shows defects in the splicing process following injection of splice-blocking morpholinos (MO; denoted sp1 and sp3). Blue arrows point to the correctly spliced mRNA, whereas red arrows indicate altered splicing products. (b) Table and scheme summarizing fezl gene structure, binding sites of morpholinos and PCR oligonucleotides and the expected mRNA size in the control and mortholino (splice-blocked) injected embryos. |
|
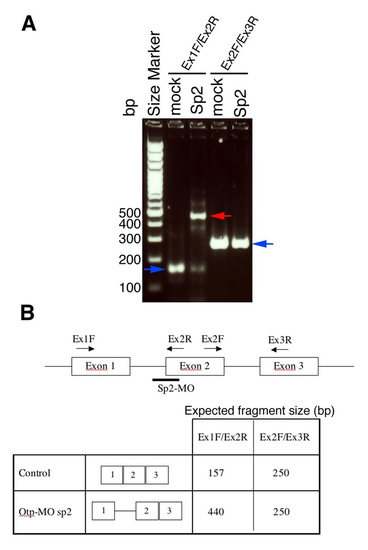
Effects of otpb-directed splice-blocking morpholino. (a) Gel electrophoresis image shows defects in the splicing process following injection of otpb morpholino (MO; denoted sp2). Blue arrows point to the correctly spliced mRNA, whereas red arrows indicate altered splicing products. (b) Table and scheme summarizing otpb gene structure, binding sites of morpholino and PCR oligonucleotide and the expected mRNA size in the control and morpholino (splice-blocking) injected embryos. |