- Title
-
Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development
- Authors
- Pyati, U.J., Webb, A.E., and Kimelman, D.
- Source
- Full text @ Development
|
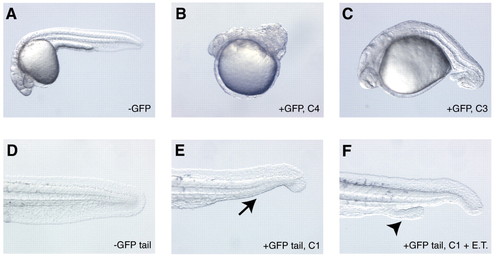
Phenotypes caused by reducing Bmp signaling at various developmental stages. Embryos from a transgenic outcross were heatshocked in an air incubator at 3 hpf (A-C) or shield stage (D-F), and the GFP-positive embryos were sorted and scored for dorsalized phenotypes at 30 hpf. Note the fully formed tail in the wild-type sibling (whole embryo in A, tail view in D). Transgenic embryos heatshocked at 3 hpf display both C4 (B) and C3 dorsalized (C) phenotypes. Note the curled trunk and tail in B and the curled tail in C. Transgenic embryos heatshocked at shield stage display either a C1 phenotype (E; arrow denotes loss of the ventral tail fin) or a C1 phenotype coupled with the formation of an ectopic tail (F; arrowhead denotes second tail). ET, ectopic tail. |
|
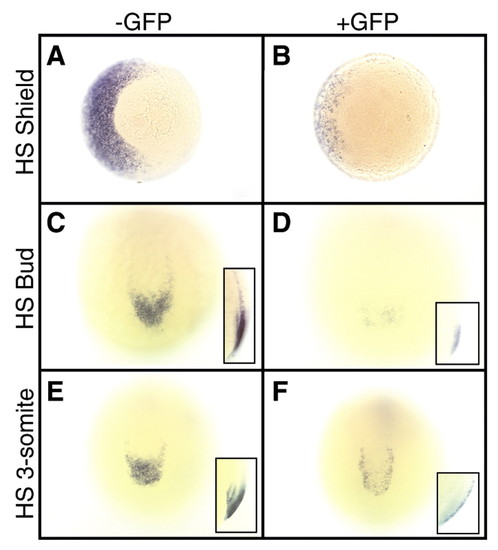
eve1 expression is dependent on Bmp signaling both during and after gastrulation. Embryos were heatshocked in an air incubator at the indicated stages, and the embryos containing the transgene were sorted by GFP fluorescence. (A,B) Vegetal views of embryos heatshocked at shield stage and assayed for eve1 expression at 70% epiboly. Note the light staining on the left (ventral) side of the transgenic embryo (B) compared with the wild-type sibling (A). (C,D) Posterior views of embryos heatshocked at bud stage and assayed for eve1 expression at the 7-somite stage. Note the darker staining of eve1 in the wild-type embryo (C), with expression extending farther anteriorly than in the transgenic embryo (D). (E,F) Posterior views of embryos heatshocked at the 3-somite stage, then assayed for eve1 expression at the 9-somite stage. Note the darker staining of eve1 in the wild-type embryo (E) than in the transgenic embryo (F). Insets in C-F show lateral views of the tailbud. HS, heatshock. EXPRESSION / LABELING:
|
|
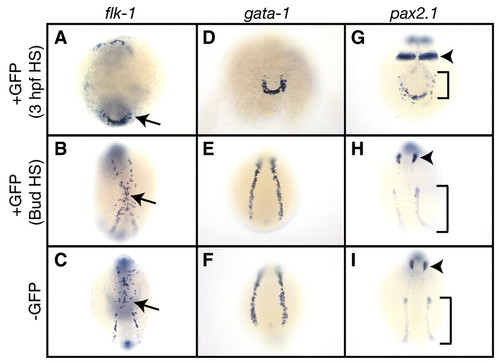
High levels of Bmp signaling are important for ventral mesoderm patterning during gastrulation but not afterward. Embryos were heatshocked in an air incubator at 3 hpf for 1 hour, or heatshocked continuously from bud stage, sorted for GFP fluorescence and fixed at the 12-somite stage. Expression of flk-1 (A-C), gata1 (D-F) or pax2.1 (G-I) was then examined by in-situ hybridization. Non-transgenic embryos heatshocked under either protocol were identical, so only a single example is shown for the ?GFP samples (C,F,I). Expression of all three markers was reduced in transgenic embryos (A,D,G) compared with wild-type siblings (C,F,I) after 3 hpf heatshocks. Gene expression was unaltered following post-gastrula heatshocks (B,E,H). Arrows in A-C denote flk-1-expressing cells. Arrowheads in G-I denote pax2.1 expression in the otic placode, while brackets indicate pax2.1 expression in the pronephric ducts and tubules. Views in A-C and G-I are dorsal, with anterior to the top. (D-F) Posterior views of embryos. EXPRESSION / LABELING:
|
|
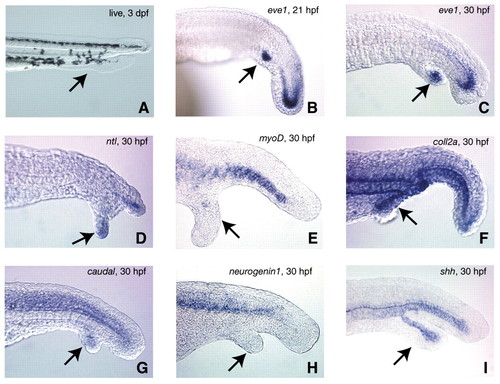
Analysis of gene expression in the ectopic tails. Embryos were heatshocked from shield to bud stage to maximize ectopic tail formation, then photographed live at 3 dpf (A), or fixed at either 21 hpf (B) or 30 hpf (C-I). Expression of eve1 (B,C), no tail (ntl; D), myod (E), collagen 2a (coll2a; F), caudal (G), neurogenin1 (H), or sonic hedgehog (shh; I). Arrows in each panel indicate the location of the ectopic tail. In A note the presence of both fin and pigment tissue in the secondary tail. In all other panels except H note the expression of each corresponding gene in the ectopic tails. ntl expression in D was localized only to the tailbud of the ectopic tail. All images except I are representative of the majority of ectopic tails examined. We observed sonic hedgehog-expressing cells in 44% (n=16) of ectopic tails. EXPRESSION / LABELING:
|