- Title
-
Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels
- Authors
- Roman, B.L., Pham, V., Lawson, N.D., Kulik, M., Childs, S., Lekven, A.C., Garrity, D.M., Moon, R.T., Fishman, M.C., Lechleider, R.J., and Weinstein, B.M.
- Source
- Full text @ Development
|
vbg mutants exhibit abnormal circulation in the absence of vessel patterning abnormalities. (A) Wild-type zebrafish at 5 dpf. (B) vbgy6 mutant at 5 dpf. Note edema in the head, pericardium, and yolk sac. This edema is associated with an abnormal circulation pattern in which most blood flows through a limited number of dilated cranial vessels, with little to no circulation through the trunk and tail (Movies 1,2; http://dev.biologists.org/supplemental/). (C,D) Schematic diagrams of the vasculature in the 2.25 dpf wild-type zebrafish head. See text for detailed explanation. (E) Dorsal anterior angiogram of a vbg+;TG(fli1:EGFP)y1 wild-type embryo at 2.25 dpf. Note the prominent central arteries (CA) and the relatively small caliber of the basal communicating artery (BCA), posterior connecting segments (PCS), basilar artery (BA), and primordial hindbrain channels (PHBC). (F) Dorsal anterior angiogram of a vbgy6;TG(fli1:EGFP)y1 mutant at 2.25 dpf. Note the limited number of patent CAs, the enlarged vessels (BCA, PCS, BA, and PHBC), and the retention of a normally transient connection (*) between the BCA and the left PHBC. (I) fli1 promoter-driven EGFP expression in the same wild-type embryo shown in E. (J) fli1 promoter-driven EGFP expression in the same vbgy6 mutant shown in F. (G) Tracing of wild-type vasculature from images shown in E,I. (H) Tracing of vbgy6 mutant vasculature from images shown in (F,J). Patent vessels in G,H are black, blue, or red; solid cords are gray. While there is no difference in CA number or pattern, almost all CAs are patent in wild-type embryos, whereas most are not patent in vbg mutants (see also gray arrows, J). AA1, first arch artery; LDA, lateral dorsal aortae; ICA, internal carotid artery; CaDI, caudal division of ICA; (A,B,D-J) Dorsal view, anterior to the left. (C) Dorsolateral view, anterior to the left. |
|
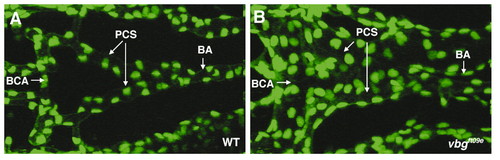
Disruption of acvrl1 increases the number of endothelial cells in the basal communicating artery (BCA) and posterior connecting segments (PCS) at 2-2.25 dpf. (A,B) Representative confocal micrograph of the central cranial vasculature of (A) a vbg+;TG(fli1;nEGFP)y7 wild-type embryo, and (B) a vbgft09e;TG(fli1;nEGFP)y7 mutant embryo. The greater than two-fold increase in endothelial cell number in the BCA/PCS in vbgft09e embryos compared to wild-type embryos is significant at P<0.00001. BA, basilar artery. Dorsal views, anterior to the left. |
|
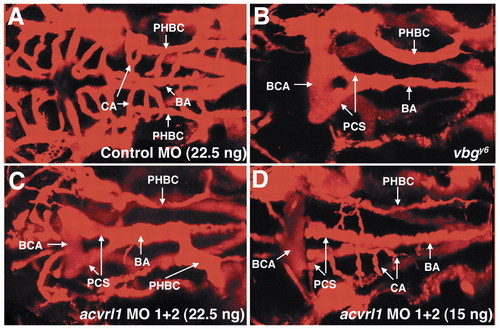
Antisense morpholino-modified oligonucleotides directed against acvrl1 phenocopy vbg mutants. Dorsal anterior angiograms of 2.25 dpf wild-type embryos injected at the 1- to 4-cell stage with 22.5 ng of a standard control morpholino (A) or 22.5 ng (C) or 15 ng (D) of a mixture of morpholinos directed against acvrl1. Note the strong resemblance of patent vessel architecture in C to that found in vbg mutants (B). The embryo shown in D is less severely affected but clearly shows dilated vessels and a decrease in the number of patent central arteries. BCA, basal communicating artery; PCS, posterior connecting segment; BA, basilar artery; PHBC, primordial hindbrain channel; CA, central artery. Dorsal views, anterior to the left. PHENOTYPE:
|
|
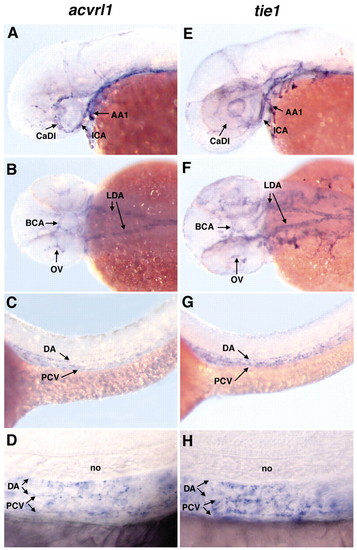
Zebrafish acvrl1 is expressed predominantly in the cranial blood vessels that become dilated in vbg mutants. Whole-mount in situ hybridization using acvrl1 (A-D) or tie1 (E-H) riboprobes on 40 hpf embryos. (A,B) Expression of acvrl1 is strongest in cranial vessels, including the first arch artery (AA1), internal carotid artery (ICA), caudal division of the internal carotid artery (CaDI), basal communicating artery (BCA) and optic vein (OV). Expression in the lateral dorsal aortae (LDA) is moderate. (C,D) Very weak acvrl1 expression is present in the dorsal aorta (DA) and posterior cardinal vein (PCV) (C, low magnification; D, high magnification). In general, tie1 is more widely expressed in cranial endothelium (E,F) than acvrl1, although relative expression in the CaDI is weaker than acvrl1 expression. In the LDA (F), DA and PCV (G, low magnification; H, high magnification), tie1 expression is qualitatively similar to acvrl1. (A,C,D,E,G,H) Lateral view, anterior to the left. (B,F) Dorsal view, anterior to the left. no: notochord. EXPRESSION / LABELING:
|
|
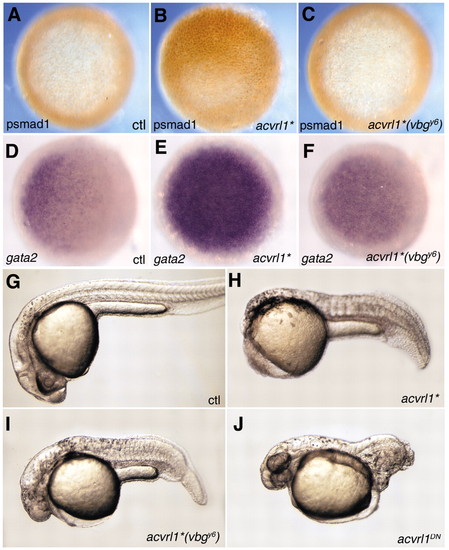
Zebrafish Acvrl1 acts through the Smad1/5/8 pathway in vivo. Shield stage embryos injected at the 1- to 4-cell stage with 5 pg mRNA encoding an activated form of Acvrl1 (acvrl1*) exhibit ectopic Smad1 phosphorylation (B) compared to uninjected controls (A). Expression of gata2 is also upregulated in acvrl1*-injected embryos (E) compared to uninjected controls (D). In contrast, 100 pg acvrl1*(vbgy6) mRNA was ineffective in inducing Smad1 phosphorylation (C), and only minimally effective in inducing gata2 expression (F). When assayed at 24-30 hpf, embryos injected with 1 pg acvrl1* mRNA (H) exhibited strong ventralization compared to uninjected controls (G), whereas those injected with 100 pg acvrl1*(vbgy6) mRNA were less severely ventralized (I). Embryos injected with 100 pg mRNA of a kinase-dead, dominant negative form of acvrl1 (acvrl1DN) exhibit strong dorsalization (J), whereas those injected with 600 pg acvrl1DN(vbgy6) were indistinguishable from wild type (data not shown). (A-F) Animal view; (G-J) lateral view, anterior to the left. |

Unillustrated author statements PHENOTYPE:
|