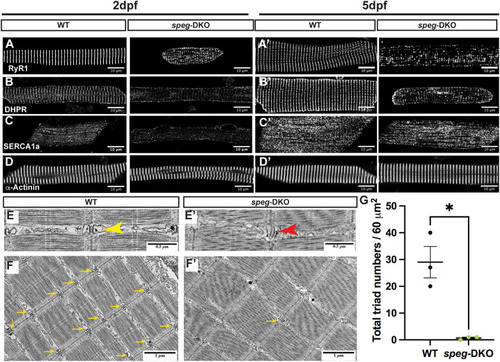
spega/b deficiency in zebrafish disrupts triad protein organization and triad ultrastructure, leading to reduced triad numbers. (A-D′) IF staining was performed on 2 dpf (A,B,C,D) and 5 dpf (A′,B′,C′,D′) isolated myofibers. Confocal images show disrupted transverse pattern of RyR1 (A,A′), DHPR (B,B′) and SERCA1a (D,D′) in speg-DKO starting from 2 dpf, while α-Actinin (D,D′) is not affected. Scale bars: 50 µm. (E-F′) Electron micrographs of 7 dpf WT and speg-DKO muscles. (E) In WT zebrafish skeletal muscle, normal triads are physically above the sarcomeric Z-disks and composed of centrally-located T-tubules flanked by terminal sarcoplasmic reticulum (yellow arrowhead). (E′) Triads in speg-DKO appear structurally disrupted, losing the obvious terminal cisternae of the sarcoplasmic membrane (tSR)/T-tubule/tSR pattern (red arrowhead). (F,F′) More importantly, the majority of sarcomeric Z-disks in speg-DKO do not have adjacent triads (yellow arrows). Scale bars: 0.5 µm (E,E′); 1 µm (F,F′). (G) The total number of triads per 60 µm2 (under an electron microscope) was significantly reduced in speg-DKO. Each dot represents the average of technical triplicates, and three biological replicates are included. Data are mean±s.e.m. Unpaired two-tailed Student's t-test: *P<0.05.
|

