Fig. 7
- ID
- ZDB-FIG-150527-18
- Publication
- Meyen et al., 2015 - Dynamic filopodia are required for chemokine-dependent intracellular polarization during guided cell migration in vivo
- Other Figures
- All Figure Page
- Back to All Figure Page
|
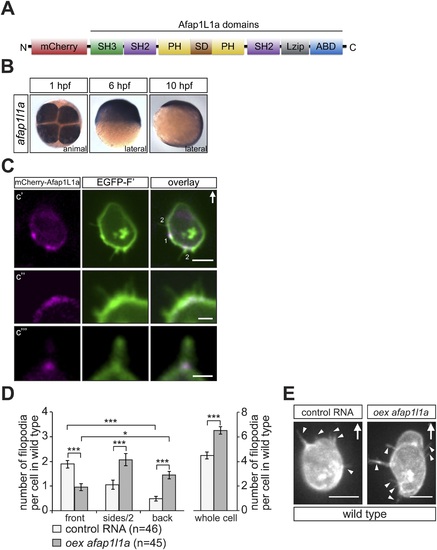
afap1l1a RNA expression, Afap1L1a protein localization and the role of the protein in filopodia formation. (A) Schematic structure of the Afap1L1a protein domains including a serine-threonine-rich substrate domain (SD) flanked by two PH domains, a leucine zipper (Lzip), an actin-binding domain (ABD), two SH2 and one SH3 domains. mCherry fluorophore was fused to the N-terminus of Afap1L1a to determine the subcellular localization of the protein. (B) Ubiquitous expression of the afap1l1a RNA in 1, 6 and 10-hpf embryos. (C) A single plane of PGCs expressing EGFP-F′ and the mCherry-Afap1L1a fusion protein reveals weak expression of the protein around the cell perimeter (C2) and a predominant strong association with the base of filopodia (C2?C4). In C2, one marks the site of a retracted filopodium, two the sites of extended filopodia and arrow indicates the direction of migration. (D) The number and distribution of filopodia in migrating PGCs overexpressing afap1l1a (dark bars) as compared with control PGCs (light bars). ?n? indicates the number of cells analysed. (E) Representative images of control (left) and of afap1l1a-overexpressing PGCs. Arrows indicate the direction of movement and arrowheads point at filopodia. Scale bars signify 10 Ám except for C3 and C4, where the scale is 3 Ám. |