Fig. 5
- ID
- ZDB-FIG-140917-43
- Publication
- Carr et al., 2014 - Characterization of the Zebrafish Homolog of Zipper Interacting Protein Kinase
- Other Figures
- All Figure Page
- Back to All Figure Page
|
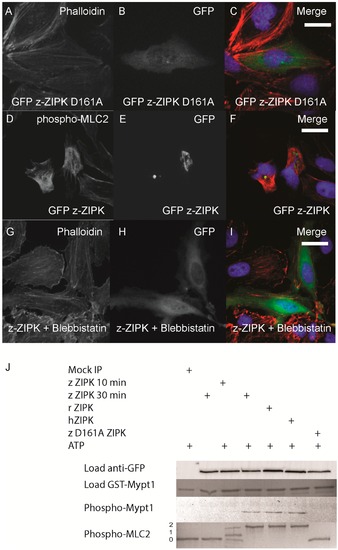
Zebrafish ZIPK controls the actin cytoskeleton by regulating type-II myosin (MLC2) phosphorylation. HeLa cells were transfected with a kinase dead D161A zebrafish GFP-ZIPK (A, B, C) and fixed and stained with DAPI and Alexa 568-phalloidin; HeLa cells were transfected with a zebrafish GFP-ZIPK (D, E, F) and immunostained using an anti-phospho myosin light chain 2 antibody, co-stained with DAPI; HeLa cells were treated with media containing either 0.1% DMSO (not shown) or 50 ÁM blebbistatin (G, H, I) for 4 h; Black and white images show phalloidin staining, while color images are a merge of DAPI (blue), GFP (green), and phalloidin (red). (J) Zebrafish, Rat and Human ZIPK immunoprecipitated from HEK 293T cells were used to phosphorylate purified GST-Mypt1 and GST-MLC2. Unphosphoryled (0), mono (1) and di-phosphorylation (2) MLC2 was detected by band shift using a phos-tag SDS-PAGE gel and stained with Coomassie. Phosphorylation of Mypt1 was detected using a phospho-specific antibody (T696). Each experiment was replicated a minimum of three times. White bar indicates a 20 μm scale bar. |