Fig. 3
- ID
- ZDB-FIG-120720-33
- Publication
- Zou et al., 2012 - Crb Apical Polarity Proteins Maintain Zebrafish Retinal Cone Mosaics via Intercellular Binding of Their Extracellular Domains
- Other Figures
- All Figure Page
- Back to All Figure Page
|
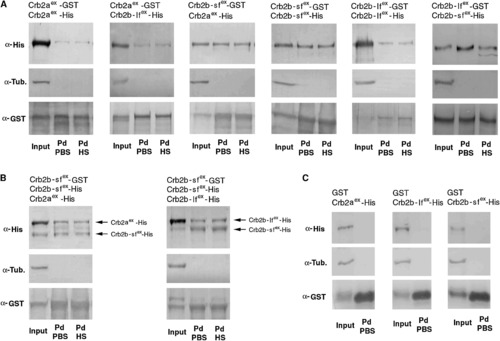
The Extracellular Domains of Crb2a, Crb2b-lf, and Crb2b-sf Mediate Homophilic and Heterophilic Physical Adhesion Various combinations (indicated above the blots) of GST and His fusions of the extracellular domains of Crb2a, Crb2b-lf, and Crb2b-sf were coexpressed in sf9 cells. The cell lysates were analyzed by GST pull-down and western blotting assays. Anti-GST blots confirmed the efficient pull down of GST fusion proteins. Anti-α-tubulin blotting indicated the absence of cytoplasmic protein contamination in the pull-down fractions (Pd). The pull-down fractions were washed either with PBS (PBS) only or with PBS as well as 1 M NaCl high-salt solution (HS). (A) Pull-down analyses of pairwise and coexpressed GST- and His-fusions indicated that the extracellular domain of Crb2a, Crb2b-lf, and Crb2b-sf physically adhered to each other both homo- and heterophilically. In addition, the adhesion was strong enough to withstand high ionic washes. (B) Competition pull-down analyses revealed that Crb2b-sfex-GST bound to Crb2b-sfex-His more efficiently than it bound to Crb2b-lfex-His or Crb2aex-His, as suggested by the higher ratios between Crb2b-sfex-His and its Crb2b-lfex-His or Crb2aex-His competitors in the pull-down fractions than in the input fractions. (C) As specificity controls, when coexpressed with Crb2aex-His, Crb2b-lfex-His, or Crb2b-sfex-His, GST itself did not pull down any Crb-His fusions, indicating that these His-fusions did not bind to GST or glutathione resin nonspecifically. |
Reprinted from Developmental Cell, 22(6), Zou, J., Wang, X., and Wei, X., Crb Apical Polarity Proteins Maintain Zebrafish Retinal Cone Mosaics via Intercellular Binding of Their Extracellular Domains, 1261-1274, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell