Fig. S3
|
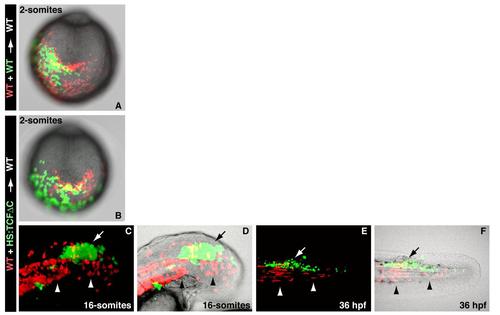
Morphogenetic defects in cells lacking Wnt signaling are not apparent until mid-somitogenesis, related to Figure 2 (A) A control experiment in which wild-type fluorescein and wild-type rhodamine cells were co-transplanted into an unlabeled wild-type host embryo and then examined at the 2-somite stage. (B) Double transplants of wild-type (red) and HS:TCFΔC (green) cells heat-shocked before gastrulation do not display noticeable differences at the 2-somite stage (A and B are posterior end-on views with dorsal to the top). (C, D) A high magnification view of a host embryo at the 16-somite stage in which wild-type (red) and HS:TCFΔC (green) cells heat-shocked before gastrulation were transplanted into the ventral margin. The majority of the wild-type cells have joined the hypoblast (arrowheads), while the HS:TCFΔC cells remain in the epiblast (arrow)(D is the image in C with the brightfield image overlaid). (E and F) At 36 hpf, the embryo from C and D shows that the wild-type cells that joined the hypoblast have given rise mostly to muscle fibers of the somites (arrowheads), while HS:TCFΔC cells that remained in the epiblast have contributed to the spinal cord (arrow) (F is the image in E with the brightfield image overlaid). |
Reprinted from Developmental Cell, 22(1), Martin, B.L., and Kimelman, D., Canonical Wnt Signaling Dynamically Controls Multiple Stem Cell Fate Decisions during Vertebrate Body Formation, 223-232, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell