- Title
-
Moderate Elevation of Homocysteine Induces Endothelial Dysfunction through Adaptive UPR Activation and Metabolic Rewiring
- Authors
- Chatterjee, B., Fatima, F., Seth, S., Sinha Roy, S.
- Source
- Full text @ Cells
|
Sub-lethally increased Hcy causes endothelial dysfunction. ( |
|
Sub-lethal HHcy reduces endothelial migration and proliferation without suppressing VEGF/VEGFR transcripts and ROS level change. ( |
|
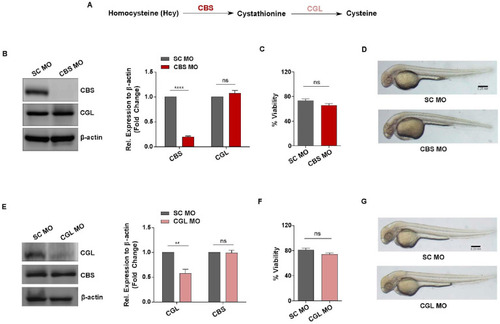
Generation of in vivo knockdown models of CBS and CGL, regulators of transsulfuration pathway involved in Hcy catabolism. ( |
|
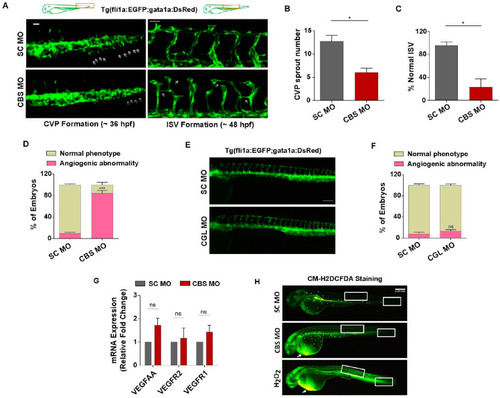
Sub-lethal HHcy causes vascular abnormality in vivo without suppressing VEGF/VEGFR transcripts and ROS level change. ( |
|
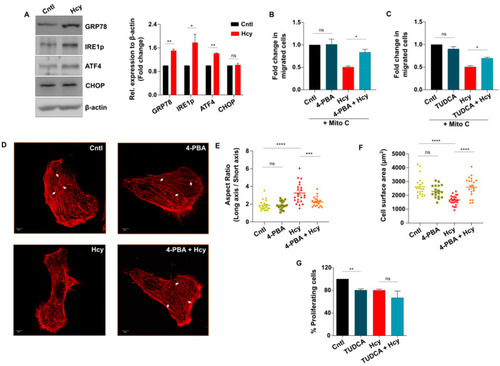
Sub-lethal HHcy-induced adaptive UPR controls endothelial migration defect. ( |
|
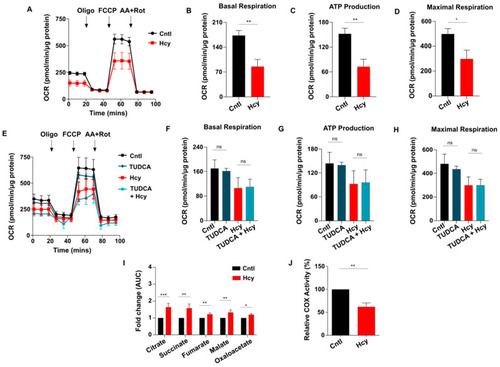
Sub-lethal HHcy linked malfunctional ETC impairs mitochondrial respiration of endothelial cells. ( |
|
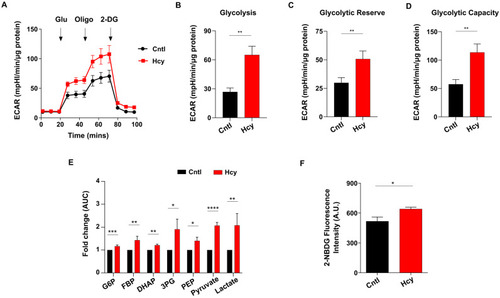
Glycolysis is elevated upon induction of sub-lethal HHcy in endothelial cells. ( |
|
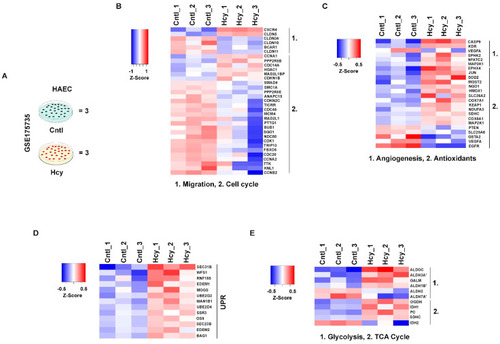
Mechanistic features of pathologically relevant Hcy exposure are conserved in adult endothelial cells. ( |