- Title
-
Spinal cords: Symphonies of interneurons across species
- Authors
- Wilson, A.C., Sweeney, L.B.
- Source
- Full text @ Front. Neural Circuits
|
A cross-species comparison of the neural basis of vertebrate movement. |
|
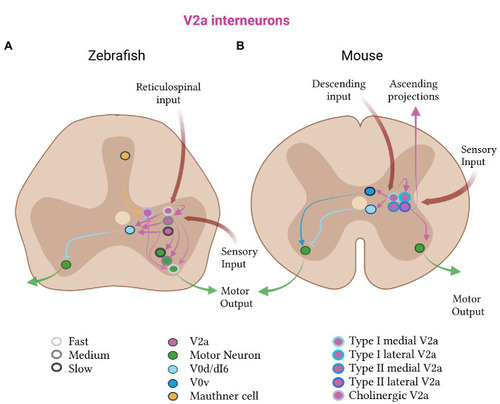
Excitatory V2a subtypes in zebrafish and mice. |
|
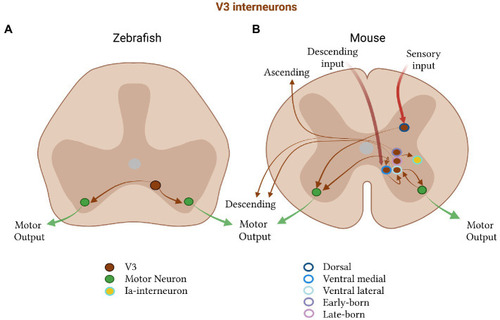
Excitatory V3 subtypes in zebrafish and mice. |
|
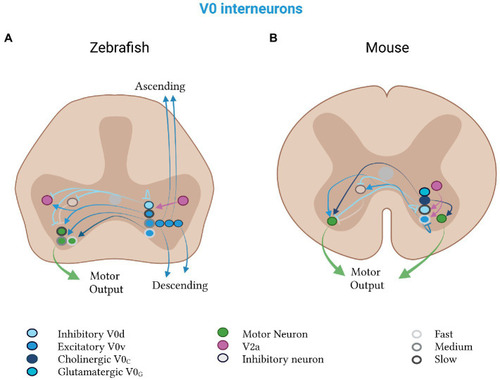
Mixed V0 subtypes in zebrafish and mice. V0d neurons (light blue) inhibit, and V0v neurons (medium blue) excite, contralateral motor neurons (green). |
|
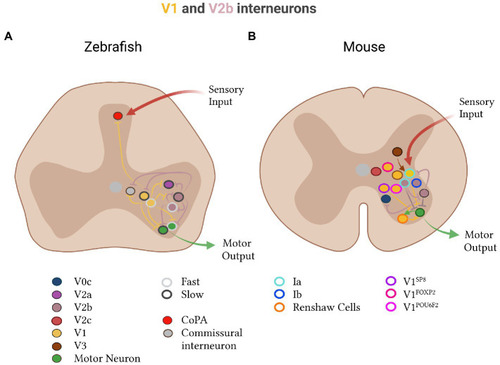
Inhibitory V1 and V2b subtypes in zebrafish and mice. |
|
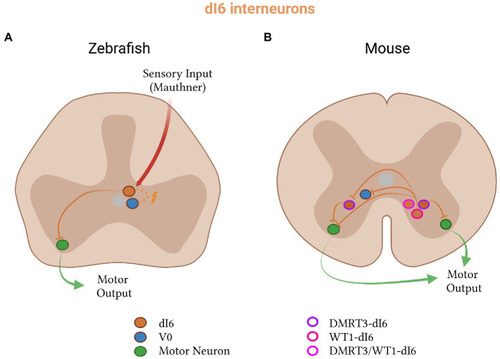
Inhibitory dI6 subtypes in zebrafish and mice. |
|
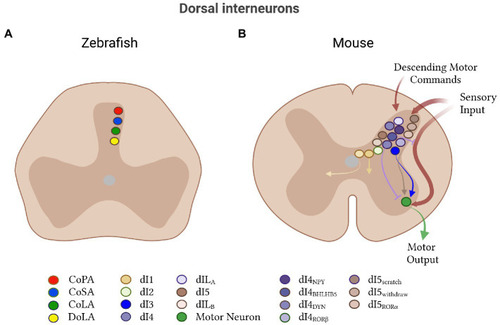
Other dorsal interneurons in zebrafish and mice. |
|
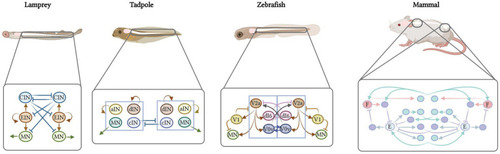
Computational models of the lamprey, tadpole, zebrafish and mammal spinal cord networks. The region of the spinal cord modeled is indicated. Far left: lamprey CPG with excitatory interneurons (EINs), inhibitory commissural interneurons (CINs), and motor neurons (MNs). Middle left: CPG model of the tadpole with the same neuron types as the lamprey plus additional ipsilateral inhibitory neurons (aINs). Middle right: CPG of the zebrafish larva at a stage when it can perform beat-and-glide swimming. Compared to the tadpole model, this model has additional contralateral excitatory neurons. Far right: model of the mammalian spinal cord showing connections between right and left rhythm generators (red: flexor unit, F; blue: extensor unit, E) at the limb level. Turquoise circles represent excitatory interneurons while purple circles represent inhibitory interneurons. |