- Title
-
GCNA Preserves Genome Integrity and Fertility Across Species
- Authors
- Bhargava, V., Goldstein, C.D., Russell, L., Xu, L., Ahmed, M., Li, W., Casey, A., Servage, K., Kollipara, R., Picciarelli, Z., Kittler, R., Yatsenko, A., Carmell, M., Orth, K., Amatruda, J.F., Yanowitz, J.L., Buszczak, M.
- Source
- Full text @ Dev. Cell
|
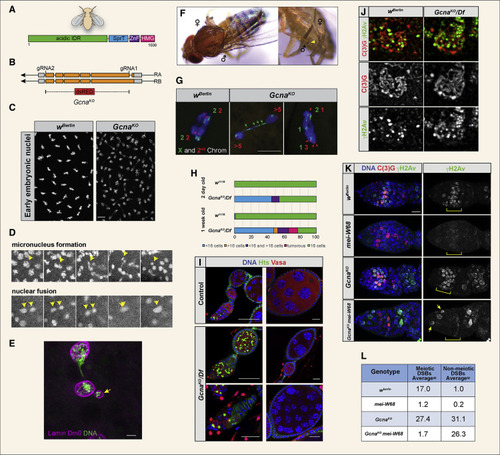
Loss of Gcna Results in Chromosome Instability in Drosophila (A) Domain organization of the Drosophila Gcna protein. (B) Drosophila Gcna gene locus showing the two isoforms and design of the GcnaKO allele with insertion of a dsRED cassette. UTRs in gray, exons in orange. (C) DAPI staining of control (wBerlin) and GcnaKO (maternal null) embryos reveals mitotic defects caused by loss of maternal Gcna. Scale bar represents 10 ?M. (D) Still images from movies of GcnaKO maternal-null embryos carrying a HistoneH2Av-mRFP transgene to visualize chromosomes. Yellow arrowheads indicate micronucleus formation (top) and nuclear fusion event (bottom). (E) Early Gcna maternal mutant Drosophila embryo stained for Lamin Dm0 (magenta) and DNA (green) to show that a nuclear envelope forms around a micronucleus (yellow arrow). Scale bar represents 5 ?m. (F) Gynandromorph phenotypes in progeny of GcnaKO females. Left, note the line of dark and light pigmentation bisecting the thorax, reflecting the presence or absence of the yellow marker carried on one of the X chromosomes. Right, sex combs are seen on one forelimb (yellow arrow) but are missing from the other, reflecting adoption of male and female fates respectively. (G) Embryonic nuclei from control (wBerlin) and GcnaKO mutant females were labeled with FISH probes to the X (green) and second (red) chromosomes. The green and red numbers refer to number of X and second chromosome-specific foci observed in each half of the dividing nuclei. Controls show chromosomes dividing equally into daughter cells. GcnaKO cells have aberrant chromosome numbers and lagging chromosomes (green arrows). Scale bar represents 5 ?m. (H and I) Quantification (H) and corresponding images (I) of Drosophila ovarian phenotypes. Hts marks the fusome; Vasa marks germ cells. Control (w1118) ovarioles contain the expected 16-cell cysts. GcnaKO/Df ovarioles have many cysts with abnormal numbers of cells, as well as tumors. The frequency of these phenotypes worsens with age. Scale bars represent 20 ?m. (J) SIM images of wBerlin and GcnaKO mutant meiotic nuclei stained for synaptonemal complex marker C(3)G (red) and DNA damage marker ?H2Av (green). (K) wBerlin, GcnaKO, mei-W860949, and GcnaKO; mei-W680949 ovarioles stained for C(3)G (red), ?H2Av (green), and DNA (DAPI, blue). Scale bar represents 10 ?m. (L) Average number of ?H2Av labeled foci per nucleus in the indicated genotypes. Ten meiotic and non-meiotic nuclei were examined per genotype. |
|
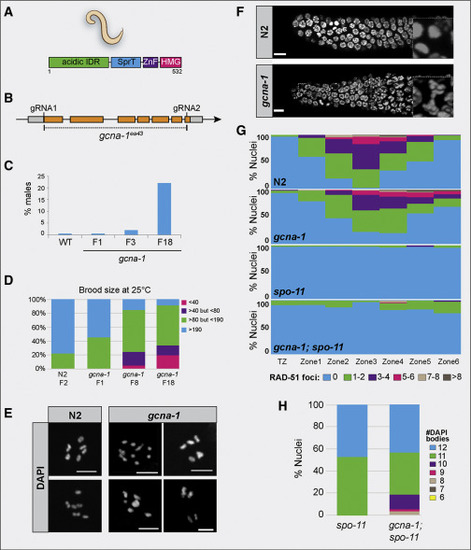
Loss of gcna-1 Results in Genomic Instability in C. elegans (A) Domain structure of C. elegans GCNA-1 protein. (B) The C. elegans gcna-1 locus detailing the design of the gcna-1(ea43) allele. (C) The HIM phenotype increases over generations (n = 12 parental worms for N2 (wild-type control) and n = 21, 21, and 34 for gcna-1 F1, F3, and F18, respectively). (D) gcna-1 mutant worms exhibit decreases in brood size with successive passaging (n = 12 parental worms for wild type, and 34 each for gcna-1 F1, F8, and F18). (E) DAPI staining of diakinesis nuclei from control (N2, wild type) and gcna-1 mutants. Controls showed the expected 6 DAPI+ bivalents; gcna-1 mutant nuclei contained mixtures of bivalents, univalents, and fused chromosomes. Scale bars represent 5 ?m. (F) Mitotic germ cells from gcna-1 mutants exhibit chromosome bridges as seen by DAPI-stained DNA. Insets are examples of nuclei in anaphase. Scale bars represent 5 ?m. (G) Quantification of meiotic (leptotene through onset of diplotene) RAD-51 foci in N2, gcna-1, spo-11, and gcna-1;spo-11 mutant C. elegans germ lines (n = 3 germlines/genotype). (H) Quantification of DAPI positive bodies in diakinesis-stage, -1 oocytes of C. elegans (spo-11, n = 20; gcna-1;spo-11, n = 42). |
|
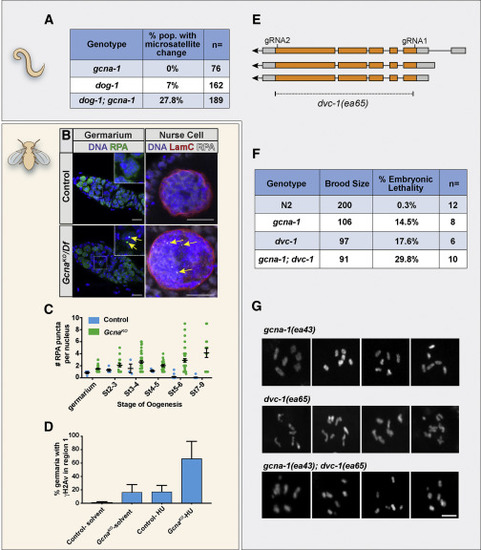
GCNA Mutant Germ Cells Experience Replication Stress (A) Frequency of microsatellite repeat length changes within the C. elegans vab-1 locus. (B and C) RPA foci (yellow arrows) accumulate in Drosophila GcnaKO germ cells. (B) Control and GcnaKO/Df Germaria (left) and nurse cell nuclei (right) stained for DNA (blue), RPA (green), and Lamin C (red) as indicated. Scale bars represent 10 ?m. (C) Quantification of RPA punctae per nucleus. 13-49 nuclei were counted per genotype per stage in triplicate. (D) Quantification of ?H2Av foci in region 1 of the Drosophila germarium in control and GcnaKO mutant germaria in response to HU treatment. 37-52 germaria were counted per genotype per stage in triplicate. Mean values are shown with standard deviation. (E) The dvc-1/Spartan locus of C. elegans showing the strategy for creating a complete null, dvc-1(ea65). (F) Brood analysis (total viable adult offspring) and embryonic lethality associated with N2, dvc-1 and gcna-1 single mutants, and dvc-1;gcna-1 double mutant worms. n > 6 broods/genotype, 3-way Kruskal?Wallis test, p < 0.05. (G) Examples of DAPI-stained, diakinesis oocytes from dvc-1, gcna-1, and dvc-1; gcna-1 mutant germ cells show chromosome fragmentation in the double mutant. Scale bars represent 5 ?m. |
|
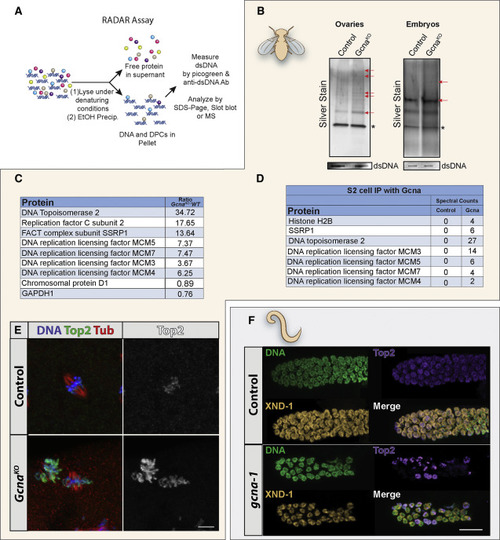
GCNA Mutants Exhibit Increased DPC Levels (A) Schematic illustrating principle of the RADAR assay. (B) RADAR was performed on Drosophila ovaries and embryos. Samples were normalized to dsDNA, separated by SDS-PAGE, and silver stained. Red arrows mark examples of differentially enhanced bands in the Gcna mutant samples. ? marks benzonase added to the prep. (C) Proteins enriched in DPCs from GcnaKO Drosophila embryos were analyzed by mass spectrometry and presented as enriched over wild-type controls. (D) List of proteins that co-immunoprecipate with Gcna expressed in Drosophila S2 cells. (E and F) Top2 is mislocalized in Gcna mutant flies and worms. (E) Early Drosophila embryos derived from control and GcnaKO females stained for DNA (blue), Top2 (green) and tubulin (Tub, red). Gray scale shows Top2 alone. Scale bar represents 5 ?m. (F) Control (N2) and gcna-1 mutant C. elegans gonads stained for DNA (green), TOP-2 (magenta), and staining control XND-1 (yellow). Scale bar represents 10 ?m. |
|
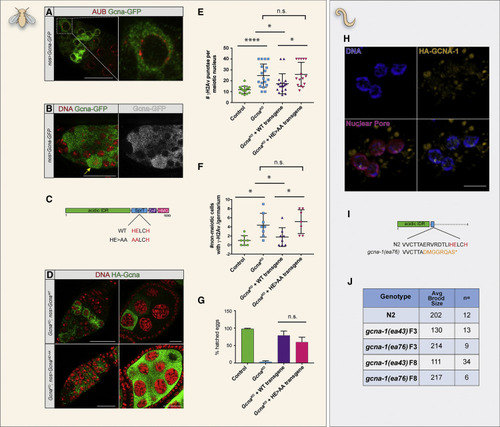
Functional Analysis of GCNA Domain Structure (A and B) Expression of a UAS-GcnaWT transgene tagged at the C terminus driven by nanos (nos)-gal4 shows prominent cytoplasmic staining. Germaria co-labeled for the Gcna-GFP transgene and (A) the nuage component, Aubergine (red) or (B) DAPI (red) to visualize DNA. Yellow arrow points to a mitotic germ cell. Scale bars represent 20 ?m. (C) Structure of the Drosophila GcnaWT and catalytic dead, GcnaHE>AA, mutant transgenes. (D) Comparison the GcnaWT and GcnaHE>AA proteins tagged at their N termini. Note the abundant GcnaHE>AA punctae in the germarium and germ cells. DNA (red), Gcna (anti-HA, green). Scale bars represent 20 ?m. (E?G) The catalytic dead transgene confers a separation-of-function phenotype and (E and F) cannot suppress the accumulation of ?H2Av foci in (E) meiotic and (F) non-meiotic nuclei from control, GcnaKO, GcnaKO; nos>GcnaWT, and GcnaKO; nos>GcnaHE>AA ovaries, but (G) can partially rescue the maternal-effect lethality conferred by loss of Gcna. Plotted are the percentages of eggs derived from females of the indicated genotypes that hatch into larvae. (E) Control (n = 16), GcnaKO (n = 18), GcnaKO; nos > GcnaWT(n = 18), GcnaKO; nos > GcnaHE>AA (n = 15). (F) n = 8 for all four genotypes. (G) Control (n = 1035), GcnaKO (n = 749), GcnaKO; nos > GcnaWT(n = 1059), GcnaKO; nos > GcnaHE>AA (n = 615). (E?G) Graphs depict mean values with standard deviation. (H) Expression of C. elegans OLLAS::GCNA-1. Germ cells are labeled for DNA (blue), GCNA-1 (anti-OLLAS, yellow), and the nuclear pore (antibody mAb414, magenta). Scale bar represents 5 ?m. (I) Structure of the C. elegans ?IDR-only?, C-terminal truncation allele, gcna-1(ea76). (J) Comparison of brood sizes from N2 control, and homozygous, F3 and F8 gcna-1(ea43) null, and gcna-1(ea76) truncation mutants. The IDR-only mutation does not exhibit the rapid transgenerational sterility seen in the null. |
|
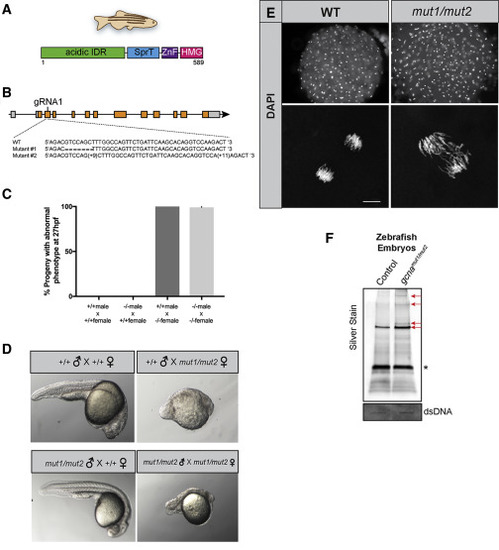
GCNA Function Is Conserved in Vertebrates (A) Domain structure of the zebrafish GCNA protein. (B) Organization of the zebrafish gcna gene showing the lesions in the mut1 and mut2 alleles. (C and D) Zebrafish embryos derived from mutant gcna mutant females exhibit profound maternal-effect defects, regardless of the paternal genotype. (C) Quantification of defects and (D) representative embryos from indicated crosses. (E) Fixed, DAPI-stained embryos derived from mutant gcna mutant females exhibit asynchronous divisions and extensive chromosome bridges during early development. For +/+M;+/+F: n = 142; -/-M; +/+F: n = 114; +/+M; -/-F: n = 173; -/-M; -/-F: n = 75. Scale bar represents 10 ?m. (F) RADAR was performed on zebrafish embryos, as described in Figures 4A and 4B. Samples were normalized to dsDNA input, separated by SDS-PAGE, and silver stained. Red arrows mark bands increased in gcna mutants. ? marks benzonase. |
Reprinted from Developmental Cell, 52(1), Bhargava, V., Goldstein, C.D., Russell, L., Xu, L., Ahmed, M., Li, W., Casey, A., Servage, K., Kollipara, R., Picciarelli, Z., Kittler, R., Yatsenko, A., Carmell, M., Orth, K., Amatruda, J.F., Yanowitz, J.L., Buszczak, M., GCNA Preserves Genome Integrity and Fertility Across Species, 38-52.e10, Copyright (2019) with permission from Elsevier. Full text @ Dev. Cell