- Title
-
Progesterone modulates microtubule dynamics and epiboly progression during zebrafish gastrulation
- Authors
- Eckerle, S., Ringler, M., Lecaudey, V., Nitschke, R., Driever, W.
- Source
- Full text @ Dev. Biol.
|
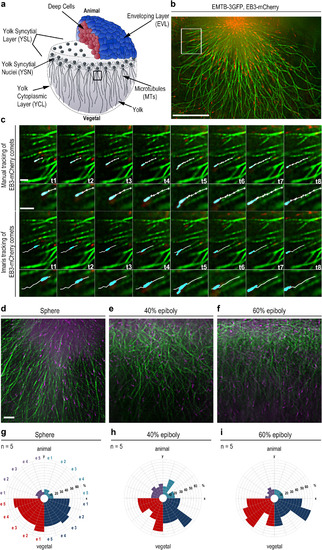
Visualization of yolk cell microtubule dynamics. (a) Wild-type embryo at sphere stage (4 hpf). Embryo images in all figures are shown in lateral views, animal at top. Black square: YCL region beneath YSL, in which microtubule growth dynamics was analyzed. (b) Live sphere stage wild-type embryo expressing EMTB-3GFP (green) and EB3-mCherry (red) imaged by TIRF microscopy in the region marked in (a). Scale bar 20 Ám. (c) Time series of microtubules of the region marked in (b). EB3-mCherry (cyan) tracking over 8 time points (1.7 s per frame). Top rows manual tracking (white, one cross per frame), bottom rows Imaris« tracking. Higher magnifications of the tracks in the small images. Both tracking methods yielded similar track lengths (manual 4.77 Ám +/? 0.18 stdev; Imaris 4.71 Ám +/? 0.05). Scale bars 2 Ám. (d-f) YCL regions of EMTB-3GFP (green) and EB3-mCherry (magenta) labeled live wild-type embryos at indicated developmental stages. Scale bar 5 Ám. (g-i) Directional distribution of EB3-mCherry tracks binned into 90 degree color coded quadrants. Each embryo (e1-e5) shown as one segment per quadrant. A significantly higher number of tracks have vegetal direction at all stages (p<0.005; Mann-Whitney test). |
|
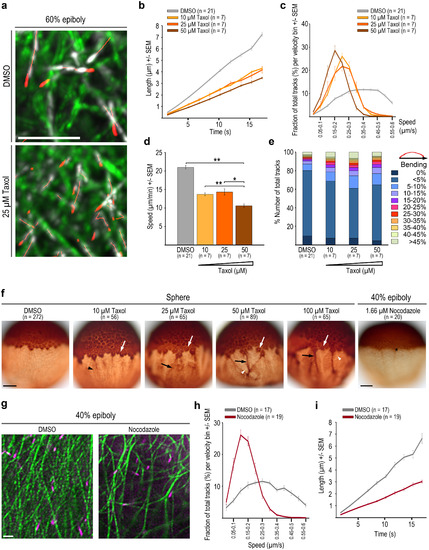
Taxol and Nocodazole affect yolk cell microtubule organization and dynamics. (a) Live imaging of YCL labeled with EMTB-3GFP (green) and EB3-mCherry (white, tracking highlighted in red) in DMSO control and Taxol treated wild-type embryos at 60% epiboly. Time series projection of 4 consecutive time points (5.1 s in total). Scale bar 5 Ám. (b-d) Microtubule growth rates (b), growth track velocity distribution (c), and average track speed (d) at 60% epiboly for DMSO control and three Taxol concentrations. Error bars - SEM. p *<0.05, **<0.005, Mann-Whitney test in d. (e) Microtubule growth track bending for DMSO control and Taxol treated embryos. (f) Sphere wild-type embryos anti-?-tubulin HRP/DAB immuno-histology. DMSO control, Taxol or Nocodazole treated embryos as indicated. Taxol: white arrows mark microtubule accumulation around MTOCs, black arrowheads mark denser YCL microtubule arrangement, black arrows mark irregular bundling of YCL microtubules upon Taxol treatment, white arrowheads mark small MT free patches. Nocodazole: black asterisk marks remaining MTOC and coherent YSN with emanating microtubules. Scale bar 100 Ám. (g) Images of live YCL region of DMSO control and Nocodazole treated wild-type embryos at 40% epiboly. Scale bar 5 Ám. (h-i) Track velocity distribution (h) and microtubule growth rates (i) at 40% epiboly in DMSO versus Nocodazole treated embryos. Error bars - SEM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
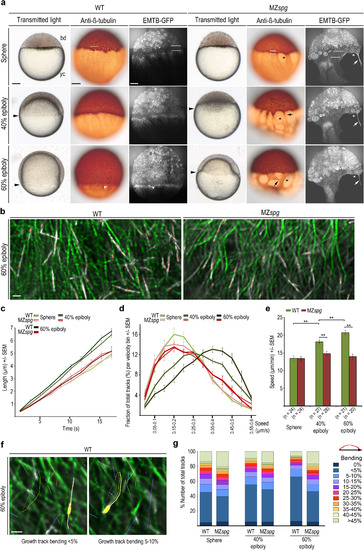
Altered yolk cell microtubule organization and dynamics in MZspgmutant embryos. (a) Wild-type (WT) and MZspg embryo live morphologies (left column: transmitted light, black arrowheads mark blastoderm margin) and microtubule organization in fixed (middle column: anti-?-tubulin immunostain) and live embryos (right column live image of EMTB-3GFP. Confocal z-projection slices). Dashed lines: YSL region between blastoderm and YCL filled with MTOCs (sphere stage); white arrowhead: wild-type YSN nucleus; black asterisks: MZspg microtubule-free patches of the yolk cell; black arrows: abnormal microtubule bundling. bd = blastoderm; yc = yolk cell. Scale bar 100 Ám. (b) Live TIRF images of YCL region labeled with EMTB-3GFP (green) and EB3-mCherry (white); wild-type (left) and mutant MZspg (right) embryos at 60% epiboly. Time-series projection of 6 consecutive time points (8.5 s in total). EB3-mCherry track is highlighted in red. Scale bar 4 Ám. (c-d) Microtubule growth rates (c) and track velocity distribution (d) in wild-type and MZspg embryos at indicated stages. Error bars - SEM, embryo numbers shown in (e). (e) Average track speed for wild-type and MZspg at indicated stages. Error bars - SEM (** p<0.005, Mann-Whitney test; at sphere N.S.). (f) Track bending. Time series projection (1.7 s per frame, 83.3 s in total) of TIRF image of live EMTB-3GFP (green) and EB3-mCherry (white) labeled YCL region of a wild-type embryo at 60% epiboly. Tracks displacement length in white, tracks color code by software based on time. Left image: yellow highlighted EB3-mCherry track with<5% bending; right image: yellow highlighted track in the 5?10% bending bin. Scale bar 2 Ám. (g) Quantification of track bending in wild-type and MZspg at indicated stages, n as in (e). EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
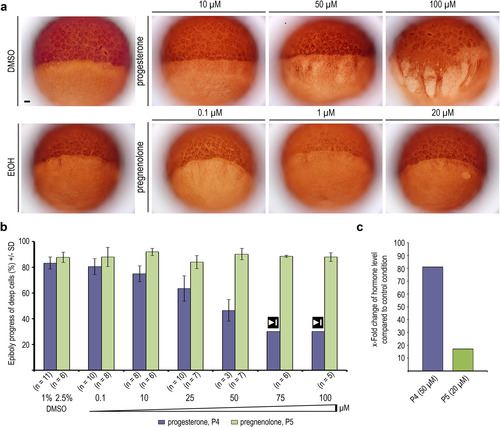
progesterone and pregnenolone effects on YCL microtubules. (a) Anti-?-tubulin immunostaining of wild-type embryos treated with progesterone or pregnenolone at indicated concentrations, and of ethanol or DMSO treated controls. Scale bar 100 Ám. (b) Quantification of progesterone and pregnenolone effects on epiboly progression, measured when control embryos reached 80% epiboly (see also supplemental Fig. S3, a and b). Embryos treated with 75 ÁM or 100 ÁM progesterone arrested gastrulation at 30?40% epiboly, marked by stop symbol (50 ? 100 ÁM: some pregnenolone precipitation occurred). Error bars - SD. (c) Progesterone and pregnenolone measured by ELISA in wild-type embryos incubated in 50 ÁM progesterone or 20 ÁM pregnenolone from 2 hpf onward. Pregnenolone levels increase>10-fold, pregnenolone levels>80-fold. EXPRESSION / LABELING:
PHENOTYPE:
|
|
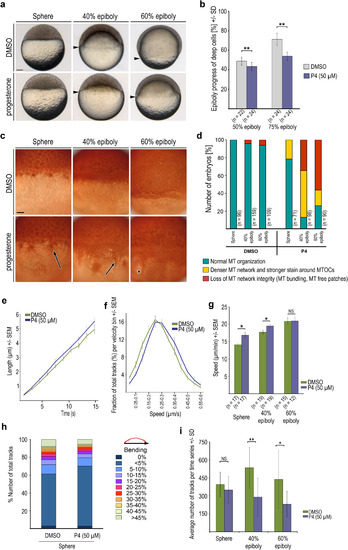
Progesterone effect on YCL microtubule organization and growth dynamics. (a) Images of live wild-type embryos treated from 2 hpf onward with progesterone (50 ÁM, lower row), and DMSO controls (upper row), photographed at indicated stages. Arrowheads: blastoderm margin. Scale bar 100 Ám. (b) Quantification of epiboly progression. P4 ? progesterone. Error bars - SD, ** p<0.005, Mann-Whitney test). (c) Anti-?-tubulin immunostaining images of the marginal region of wild-type DMSO control and 50 ÁM progesterone treated embryos fixed at indicated stages. Arrows mark microtubule accumulation around the MTOCs and increased density of microtubule bundles. Asterisk marks disorganized YCL microtubule network with microtubule free patch. Scale bar 50 Ám. (d) Quantification of progesterone (P4) induced yolk cell microtubule network organization defects. Phenotypic classes see text for details. (e, f) Microtubule growth rates (e) and track velocity distribution (f) at sphere stage for DMSO control and progesterone (P4) treated wild-type embryos. Error bars - SEM, embryo numbers shown in (g). (g) Average track speed for DMSO control and progesterone treated wild-type embryos at indicated stages. Error bars indicate SEM (* p<0.05, Mann-Whitney test; at 60% epiboly NS). (h) Quantification of track bending in DMSO control and progesterone treated embryos at sphere stage, n as in (6 g). (i) Average numbers of EB3-mCherry tracks per time series at indicated stages of DSMO control or progesterone (P4) treated wild-type embryos. Error bars - SD. (*p<0.05; ** p<0.005; Mann-Whitney test; n as in 6 g). PHENOTYPE:
|
|
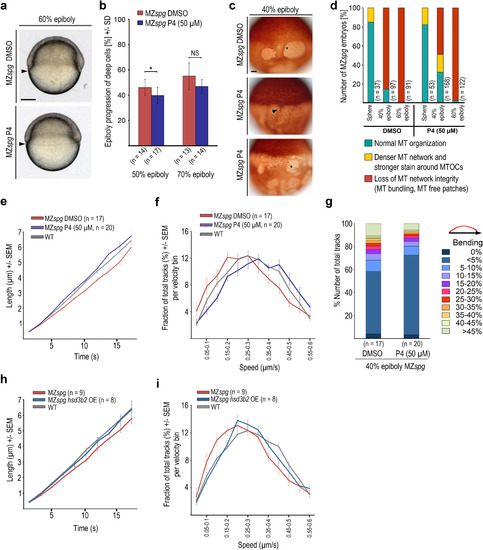
Progesterone treatment partially rescues microtubule growth dynamics in MZspg. (a) Live DMSO or progesterone (P4, 50 ÁM) treated MZspg embryos at 60% epiboly. Scale bar 100 Ám. (b) Epiboly progression of DMSO or progesterone (P4, 50 ÁM) treated MZspg embryos at indicated stages. Error bars - SD. (* p<0.05 Mann-Whitney test). (c) Anti-?-tubulin immunostained DMSO or progesterone (P4, 50 ÁM) treated MZspg embryos at 40% epiboly. Arrowhead marks microtubule accumulation around the MTOCs and denser appearance of microtubule arrays, asterisks mark loss of microtubule network integrity with microtubule free patches. Scale bar 50 Ám. (d) Quantification of microtubule phenotypes observed in experiment shown in panel (c). Classification of phenotypes as in Fig. 6d. (e, f) Microtubule growth rates (e) and track velocity distribution (f) at 40% epiboly stage for DMSO control and progesterone (50 ÁM) treated MZspg embryos. Wild-type data at 40% epiboly from Fig. 3c and d. Error bars - SEM. (g) Microtubule track bending in DMSO control and progesterone treated MZspg embryos at 40% epiboly. (h, i) Microtubule growth rates (h) and track velocity distribution (i) at 40% epiboly for MZspg control and hsd3b2 mRNA injected MZspg embryos. Wild-type data at 40% epiboly from Fig. 3c and d. Error bars - SEM. PHENOTYPE:
|
|
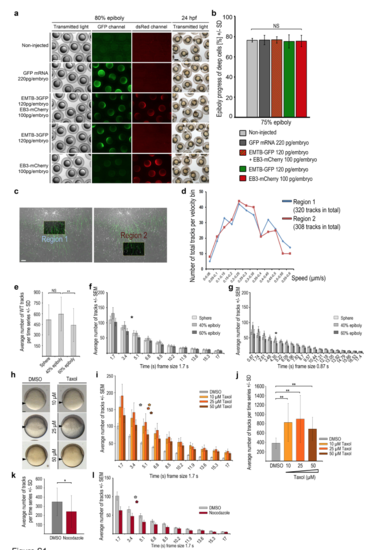
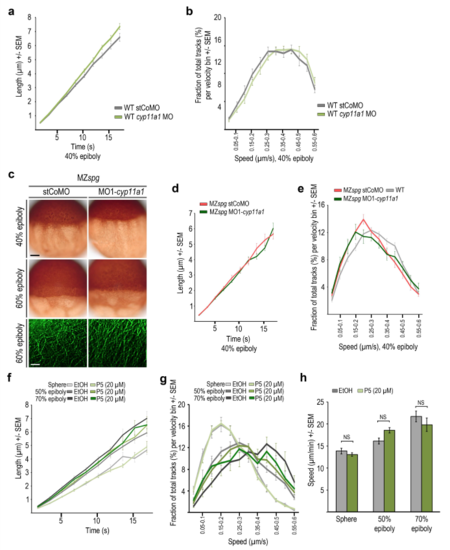
Measurements of microtubule dynamics in wild-type embryos and embryos treated with microtubule interfering drugs (a, b) EMTB-3GFP and EB3-mCherry overexpression did not impair epiboly progression. (a) Comparison of live wild-type embryos at 80% epiboly, non-injected or injected with mRNAs encoding GFP, EMTB-3GFP plus EB3-mCherry, EMTB-3GFP alone, or EB3-mCherry alone (rows from top to bottom), imaged with transmitted light (left column), for GFP- (middle column), or dsRedfluorescence (right column). Live non-injected or mRNA injected wild-type embryos at 24 hpf imaged with transmitted light (right column) reveal normal embryogenesis of labeled embryos. Scale bar 500 ?m. (b) Quantification of epiboly progression in experiment shown in (a). Error bars indicate SD. Epiboly progression of mRNA injected embryos was not significantly (NS) different compared to non-injected control embryos (Mann-Whitney test) (non-injected: n = 9 embryos, mRNA injected: GFP n = 9, EMTB-3GFP + EB3-mCherry n = 8, EMTB-3GFP n = 10, EB3-mCherry n = 9). (c, d) EB3-mCherry comet number or track distribution are not biased by minor differences in positioning the analysis frame in the YCL. Manually defined areas might slightly differ in their position in the YCL, potentially influencing microtubule dynamics measurements. Therefore, we compared analysis regions located right beneath an MTOC with those positioned more vegetally within the same embryo. (c) Same TRIF images of live wild-type YCL region at sphere labeled with EMTB-3GFP (green) and EB3-mCherry (white). Left image: square marks highlighted ?Region 1? just below the most vegetal MTOC. Right image: square marks highlighted ?Region 2? at a more vegetal position. Region size each 24 ?m x 15 ?m. Selected image of a time series (1.7 s per frame, 83.3 s in total). Scale bar 5 ?m. Both regions contained a similar number of tracked EB3-mCherry comets in each analyzed embryo. (d) Growth speed of EB3-mCherry tracks present in regions 1 or 2 of images shown in (c) was binned into speed intervals (bin size 0.05 ?m/s) and the number of tracks in each bin plotted. Track velocity distributions were not significantly different between the two analyzed regions. (e) Quantification of the average number of wild-type EB3-mCherry tracks at indicated stages. Error bars indicate SD. Same dataset analyzed as listed in Fig. 3c. Track numbers are not significantly (NS) different between sphere and 40% epiboly, but significantly reduced from 40% to 60% epiboly (p < 0.05, Mann-Whitney test). (f) Distribution of duration of individual tracks with 1.7 s per frame recording. The average number of EB3-mCherry tracks with a given duration of frames plotted over track duration (x-axis determined by frame time intervals) at indicated stages. Asterisk marks average half-life of the tracks during the time series, 83.3 s in total (calculated with ln(2)/? = 4.1 s; decay constant ? taken from exponential trend line equation calculated by Excel). At average half-life, the quantity of tracks fell to one half of its initial value (average number of tracks at time point of average half-life: sphere 217 tracks, 40% epiboly 241 tracks, 60% epiboly 198 tracks). Error bars indicate SEM. Same dataset analyzed as in Fig. 3c. (g) Distribution of duration of individual tracks with 0.87 s per frame recording. The average number of EB3-mCherry tracks with a given duration of frames plotted over track duration (x-axis determined by frame time intervals) at indicated stages. Asterisk marks average half-life of the tracks during the time series, 83.3 s in total (calculated with ln(2)/? = 4.35 s; decay constant ? taken from exponential trend line equation calculated by Excel). At average half-time the quantity of tracks fell to one half of its initial value (average number of tracks at time point of average half-life: sphere 261 tracks, 40% epiboly 307 tracks, 60% epiboly 217 tracks). Error bars indicate SEM. Same dataset analyzed as in Fig. 3c. Data in Figures S2b and c reveal that the measurement of track duration is not affected by frame duration in the range of recording frequencies used. (h) Live images at 50% epiboly of wild-type embryos treated from 2 hpf onward with DMSO (left column) or Taxol at 3 concentrations (right column). Arrowheads mark blastoderm margin. Lateral view, animal to the top. Scale bar 100 ?m. (i) Distribution of duration of individual EB3-mCherry tracks per frame for recordings of DMSO controls (grey bars) or Taxol treated wild-type embryos (3 concentrations: yellow, orange, brown bars) at 60% epiboly. Tracks with a given duration of frames plotted over track duration (x-axis determined by time intervals). Color coded asterisks mark average half-life of the tracks during the time series (calculated with: DMSO: ln(2)/? = 4 s; Taxol: 10 ?M: ln(2)/? = 5.6 s; 25 ?M: ln(2)/? = 5.4 s; 50 ?M: ln(2)/? = 5.9 s. Each decay constant ? was taken from exponential trend line equation calculated by Excel). Error bars indicate SEM. Same dataset analyzed as in Fig. 2b. (j) Quantification of average EB3-mCherry track number per time series of DMSO and Taxol treated wild-type embryos at 60% epiboly. Error bars indicate SD. Same dataset analyzed as in Fig. 2b. A fixed area (41.7 x 31.26 ?m) was analyzed for all conditions. Differences in track number are statistically significant (Mann-Whitney test) between DMSO and each tested Taxol concentrations (p < 0.005 for 10 ?M and 50 ?M; p < 0.05 for 25 ?M). (k) Quantification of the average EB3-mCherry track number per time series of DMSO or Nocodazole treated wild-type embryos at 40% epiboly. Error bars indicate SD. Same dataset analyzed as in Fig. 2h. Difference of track number between DMSO and Nocodazole treated embryo was significant (p < 0.05, Mann-Whitney test). (l) Distribution of individual EB3-mCherry tracks per frame recording of DMSO (grey bars) or Nocodazole (red bars) treated wild-type embryos at 40% epiboly. Tracks with a given duration of frames plotted over track duration (x-axis determined by time intervals). Color coded asterisks mark average half-life of the tracks during the time series (calculated with: DMSO: ln(2)/? = 3,7 s; Nocodazole: ln(2)/? = 3,9 s. Each decay constant ? was taken from exponential trend line equation calculated by Excel). Error bars indicate SEM. Same dataset analyzed as in Fig. 2h. |
|
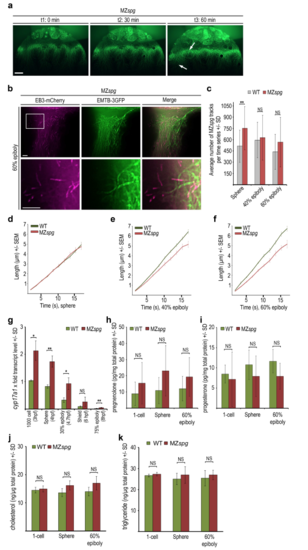
Analysis of YCL microtubule dynamic behavior and steroidogenic pathways in MZspg mutant embryos (a) Live images of a mutant MZspg embryo during epiboly progression. Embryo injected with EMTB- 3GFP mRNA at 1-cell stage. Selected images of a time series, timepoints t1-t3 correspond to: t1 30% epiboly (0 min), t2 50% epiboly (30 min), t3 shield (60 min). Arrows mark developing alterations of abnormal microtubule arrays in between regions with wild-type like appearance. Confocal zprojection, lateral view, animal to the top, scale bar 50 ?m. (b) TIRF time series projection (5 frames, 8.5 s in total) of MZspg mutant embryo YCL region labeled with EMTB-3GFP (green) and EB3-mCherry (magenta) revealing defects in microtubule assembly at border between thickened microtubule bundles and microtubule devoid zone (upper row). Lower row shows magnification of the region marked by the square. Lateral view, animal to the top, scale bar 5 ?m. (c) Quantification of the average number of EB3-mCherry tracks at indicated stages of wild-type and MZspg embryos. Error bars indicate SD. Number of EB3-mCherry comets in the mutant MZspg YCL with 753 (SD 287) at sphere, 628 (SD 300) at 40% epiboly, and 568 (SD 331) at 60% epiboly. Differences of track numbers between sphere, 40% and 60% epiboly in MZspg were not significant (Mann-Whitney test). Comparing MZspg and wild-type, differences were statistically significant only at sphere (p < 0.005; Mann-Whitney test). (d-f) The speed of microtubule growth plotted as EB3-mCherry track length (?m) over track duration (s) at sphere (d), 40% epiboly (e), and 60% epiboly (f) for MZspg compared to wild-type embryos. Error bars indicate SEM. Same dataset analyzed as in Fig. 3c. (g) Relative transcript levels of cyp17a1 determined by qRT-PCR for stages from 1000-cell to 75% epiboly for wild-type (grey bars) and MZspg (red bars) embryos. Expression levels normalized to wild-type 1000-cell stage. Error bars indicate SD (n = 3). Differences of expression levels between wild-type and MZspg were statistically analyzed (t-test): p < 0.005: sphere, 75% epiboly; p < 0.05: 1000-cell, 30% epiboly; NS: shield. (h, i) Levels of pregnenolone (h) and progesterone (j) quantified by ELISA in wild-type and MZspg embryo lysates at indicated stages. Error bars indicate SD (n = 6). At 60% epiboly the calculated progesterone content per embryo was wild-type 1.2 pg (SD 0.71), MZspg 0.78 pg (SD 0.58) and the pregnenolone content wild-type 1.09 pg (SD 0.74), MZspg 2.49 pg (SD 1.85). Statistical test (Mann- Whitney test) did not reveal significant differences between wild-type and MZspg at each stage. (j, k) Levels of cholesterol (j) and triglycerides (k) quantified by colorimetric enzyme assay of embryonic lysates at indicated stages. Error bars indicate SD (n = 3; exception: triglyceride for MZspg sphere n = 2). Differences in cholesterol and triglyceride levels between wild-type and MZspg embryos were statistically not significant (NS, Mann-Whitney test) |
|
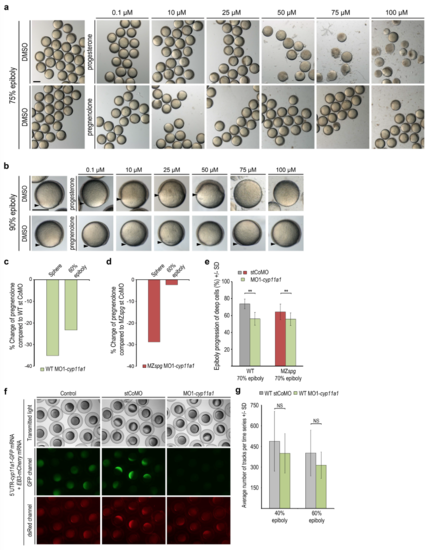
Live phenotypes of progesterone or pregnenolone treated wild-type embryos and effects of reduction of pregnenolone level by MO1-cyp11a1 injection on epiboly and microtubules (a) Overview images show groups of live treated embryos at age when wild-type siblings reached 75% epiboly. Progesterone experiment (upper row): DMSO 1% control or indicated progesterone concentrations; 75 ?M and 100 ?M progesterone treated embryos arrested gastrulation at 30-40% epiboly and disintegrated. pregnenolone experiment (lower row): DMSO (2.5%) control or indicated pregnenolone concentrations. Embryos were indistinguishable from controls at all pregnenolone concentrations. Scale bar 500 ?m. (b) Images of single live wild-type embryos from experiment shown in (a) at age when untreated wildtype siblings reached 90% epiboly. Arrowheads mark blastoderm margin. Lateral view, animal to the top, scale bar 500 ?m. (c, d) MO1-cyp11a1 injection reduced the pregnenolone level in embryos at sphere and 60% epiboly compared to stCoMO injection. Pregnenolone levels were measured by ELISA in wild-type (c) or MZspg (d) embryonic lysates (n=1). (e) Quantification of epiboly progression when control injected wild-type embryos reached 70% epiboly. stCoMO injections: wild-type: grey bar, n = 10, MZspg: red bar, n = 23. MO1-cyp11a1 injections: wild-type: green bar, n = 11; MZspg: green bar, n = 17. Error bars indicate SD. Epiboly progression of MO1-cyp11a1 injected embryos was significantly delayed compared to controls (p < 0.005, Mann-Whitney test). (f) We confirmed MO1-cyp11a1 functionality by knock-down of GFP reporter expression. Specificity and efficiency of MO1-cyp11a1 against the translation start side of cyp11a1 has been tested by coinjection of a pCS2+ GFP reporter construct, which harbors the cyp11a1 morpholino target sequence (AAGTAGAGAGAGAGAGTGTGATGGC) in frame fused to ATG-deleted GFP coding sequence. The GFP-reporter and, serving as control, EB3-mCherry mRNAs (50 pg/embryos each) were injected without morpholino (left column), with stCoMO (4 ng/embryo, middle column), or with MO1- cyp11a1 (4 ng/embryo, right column). Fluorescence (GFP: middle row; dsRed: lower row) was examined at epiboly stages. GFP reporter expression was eliminated upon co-injection of MO1- cyp11a1, but not in controls, while EB3-mCherry fluorescence was present in all samples. (g) Quantification of average EB3-mCherry track number at 40% and 60% epiboly for stCoMO, or MO1-cyp11a1 injected wild-type embryos. Error bars indicate SD (40% epiboly: stCoMO n = 16 MO1-cyp11a1 n = 18; 60% epiboly: stCoMO n = 15 MO1-cyp11a1 n = 15). Mann-Whitney test: differences NS. |
|
Effects of cyp11a1 knockdown and pregnenolone treatment on wild-type and MZspg YCL microtubule dynamics (a) The speed of microtubule growth at 40% epiboly plotted as EB3-mCherry track length (?m) over track duration (s) for stCoMo or MO1-cyp11a1 injected wild-type embryos. Morpholino injections were at 1-cell stage. Error bars indicate SEM (stCoMO n = 16; MO1-cyp11a1 n = 18). (b) The microtubule growth speed was binned into speed intervals (bin size = 0.05 ?m/s) at 40% epiboly for stCoMO or MO1-cyp11a1 injected wild-type embryos. Same dataset analyzed as in (a). Error bars indicate SEM. (c) Microtubule organization of stCoMO (left column) or MO1-cyp11a1 (right column) injected MZspg embryos at indicated stages. Upper two rows show anti-▀-tubulin immunostaining. Lateral view, animal to the top, scale bar 100 ?m. Bottom row shows YCL images of live embryos labeled with EMTB-3GFP. Scale bar 5 ?m. (d) The speed of microtubule growth at 40% epiboly plotted as EB3-mCherry track length (?m) versus track duration (s) for stCoMO or MO1-cyp11a1 injected MZspg embryos. Error bars indicate SEM (stCoMO n = 12; MO1-cyp11a1 n = 12). (e) The speed of microtubule growth at 40% epiboly was binned into speed intervals (bin size = 0.05 ?m/s) for stCoMO or MO1-cyp11a1 injected MZspg embryos. Error bars indicate SEM. Same dataset analyzed as in (f). (f) Quantification of the speed of microtubule growth at sphere, 50% epiboly, and 70% epiboly plotted as EB3-mCherry track length (?m) over track duration (s) for control or pregnenolone (20 ?M) treated wild-type embryos. Error bars indicate SEM (experimental controls: sphere: n = 11 embryos; 50% epiboly: n = 11; 70% epiboly: n = 8; pregnenolone treatment: sphere: n = 11, 50% epiboly n = 12; 70% epiboly: n = 8). (g) The speed of microtubule growth at sphere, 50% epiboly, and 70% epiboly was binned into speed intervals (bin size = 0.05 ?m/s) for control and pregnenolone treated wild-type embryos. Error bars indicate SEM. Same dataset analyzed as in (f). (h) Average speed of microtubule growth of control or pregnenolone treated wild-type embryos, stages as indicated. Error bars - SEM. Same dataset analyzed as in (f). Data revealed no significant differences (Mann-Whitney test) of microtubule growth speed between control and pregnenolone treated embryos. |
|
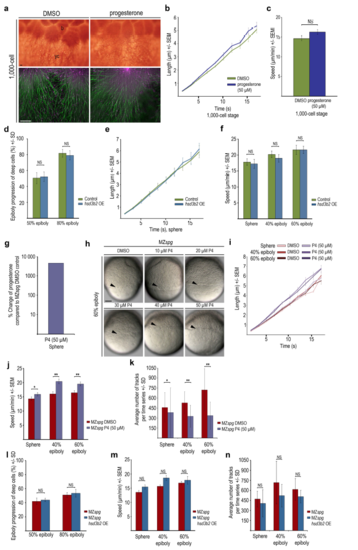
Yolk cell microtubule structure and dynamics in progesterone treated or hsd3b2 overexpressing embryos (a) YCL region of DMSO or progesterone (50 ?M) treated wild-type embryos at 1000-cell stage (3 hpf). Upper row: Anti-▀-tubulin immunostaining show marginal blastomeres (= b) fusing with yolk cell (= yc). Lower row: Live TIRF images of EMTB-3GFP (green) and EB3-mCherry (magenta) labeled embryos. Lateral view, animal to the top, scale bar 10 ?m. (b) Microtubule growth at 1000-cell plotted as EB3-mCherry track length (?m) over track duration (s) for DMSO control and progesterone (50 ?M) treated wild-type embryos. Error bars indicate SEM (DMSO n = 9; progesterone n = 11). (c) Average speed of microtubule growth at 1000-cell stage for DMSO and progesterone (50 ?M) treated wild-type embryos. Error bars indicate SEM. Same dataset analyzed as in (b). Microtubule growth speed difference between DMSO and progesterone treated embryos was not significant (Mann- Whitney test). (d) Quantification of epiboly progression of control or hsd3b2 mRNA injected wild-type embryos at indicated stages. Error bars indicate SD (control experimental series: 50% epiboly n = 16 embryos, 80% epiboly n = 10; hsd3b2 mRNA injection experimental series: 50% epiboly n = 18, 80% epiboly n = 12). Differences between control and hsd3b2 mRNA injected embryos were not significant (Mann- Whitney test). OE = overexpression. (e) Speed of microtubule growth at sphere plotted as EB3-mCherry track length (?m) over track duration (s) for control and hsd3b2 mRNA injected wild-type embryos. Error bars indicate SEM (control n = 8; hsd3b2 mRNA n = 9). OE = overexpression. (f) Average speed of microtubule growth tracks at indicated stages for control and hsd3b2 mRNA injected wild-type embryos. Error bars indicate SEM (controls: sphere n = 8, 40% epiboly n = 9, 60% epiboly n = 9; hsd3b2 mRNA injections: sphere n = 9; 40% epiboly n = 10; 60% epiboly n = 10). Statistical analysis revealed no significant differences between control and hsd3b2 mRNA injected embryos (Mann-Whitney test). OE = overexpression. (g) Incubation of MZspg embryos in 50 ?M progesterone from 2 hpf onwards increases progesterone levels more than tenfold when extracts are prepared at sphere stage. Concentration of progesterone quantified by ELISA. (h) Images of live MZspg embryos at 60% epiboly treated with indicated progesterone concentrations and DMSO control. Arrowheads mark blastoderm margin. Lateral view, animal to the top, scale bar 100 ?m. (i) The speed of microtubule growth at indicated stages plotted as EB3-mCherry track length (?m) over track duration (s) for MZspg embryos in DMSO controls or upon or progesterone (50 ?M) treatment. Error bars indicate SEM (sphere: DMSO n = 17; progesterone n = 18, 40% epiboly: DMSO n = 17; progesterone n = 20, 60% epiboly: DMSO n = 15; progesterone n = 17). (j) Average speed of microtubule growth at indicated stages for MZspg embryos in DMSO controls or upon progesterone treatment. Error bars indicate SEM. Same dataset analyzed as in (i). Progesterone induced an increase in average microtubule growth speed at sphere stage (15.9 ?m/min SEM 0.5 treated versus 14.3 ?m/min SEM 0.7 control). At 40% epiboly (20.4 ?m/min, SEM 0.8 treated versus 16.0 ?m/min, SEM 0.7 control) and 60% epiboly (19.5 ?m/min SEM 0.7 treated versus 16.4 ?m/min SEM 0.75, control) the increase was even stronger. Differences of microtubule growth speed between MZspg control and progesterone treated embryos were significant (p < 0.05 at sphere; p < 0.005 at 40% epiboly and 60% epiboly; Mann-Whitney test). (k) The average number of EB3-mCherry tracks in a defined area at indicated stages of MZspg embryos treated with DMSO or progesterone. Error bars indicate SD. Same dataset analyzed as in (i). Difference in track number between control and progesterone treated embryos was significant (p < 0.05 at sphere; p < 0.005 at 40% epiboly and 60% epiboly; Mann-Whitney test). (l) Quantification of epiboly progression at 50% and 80% epiboly of control or hsd3b2 mRNA injected MZspg embryos. Error bars indicate SD (50% epiboly: control n = 13, hsd3b2 mRNA n = 14; 80% epiboly: control n = 11, hsd3b2 mRNA n = 12). Data revealed no significant differences between control and hsd3b2 mRNA injected embryos. OE=overexpression (Mann-Whitney test). (m) Average speed of microtubule growth at indicated stages of control or hsd3b2 mRNA injected MZspg embryos treatment. Error bars indicate SEM (sphere: control n = 7; hsd3b2 mRNA n = 8, 40% epiboly: control n = 9; hsd3b2 mRNA n = 8, 60% epiboly: control n = 8; hsd3b2 mRNA n = 10). Data revealed no significant differences between control and hsd3b2 mRNA injected embryos (Mann- Whitney test). (n) Average number of EB3-mCherry tracks at indicated stages of control or hsd3b2 mRNA injected MZspg embryos. Error bars indicate SD. Same dataset analyzed as in (m) Data revealed no significant differences between control and hsd3b2 mRNA injected embryos (Mann-Whitney test). |
Reprinted from Developmental Biology, 434(2), Eckerle, S., Ringler, M., Lecaudey, V., Nitschke, R., Driever, W., Progesterone modulates microtubule dynamics and epiboly progression during zebrafish gastrulation, 249-266, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.