- Title
-
Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress
- Authors
- Scheid, L.M., Mosqueira, M., Hein, S., Kossack, M., Juergensen, L., Mueller, M., Meder, B., Fink, R.H., Katus, H.A., Hassel, D.
- Source
- Full text @ Cardiovasc. Res.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
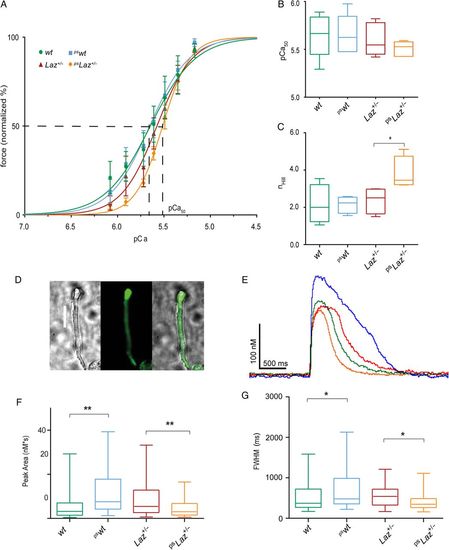
Laz affects acto-myosin cooperativity and impairs calcium handling. (A) pCa-force relationship, normalized to maximal force (at pCa 4.5). The sigmoidal curves were further analysed for pCa50 and nHill. (B) Quantification of pCa50 from the pCa-force relationship revealed no significant differences. (C) nHill was significantly increased for pslaz+/- compared with wt, pswt, and laz+/- (P < 0.05; N = 5). (D) Intracellular Ca2+-transients were analysed in isolated ventricular cardiomyocytes loaded with Fluo4-AM. Representative brightfield (left) and corresponding fluorescent (middle) and merged (right) images of a single ventricular adult zebrafish cardiomyocyte (scale bar 20 Ám). (E) Representative Ca2+-transient traces recorded from wt, pswt, laz+/-, and pslaz+/-. (F) Peak area and (G) full-width half-maximum (FWHM) obtained from the analyses of Ca2+-transients. Data in (F) and (G) were statistically analysed by using ANOVA with Bonferonni post hoc pairwise multiple comparisons *P < 0.05; **P < 0.01; in A-C: N = 5; in E-G: n = 90 cells. |
|
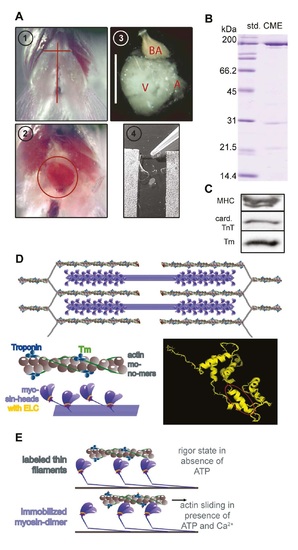
Preparation of actin and myosin for IVMA. (A) Horizontal skin section between the gills and a longitudinal section above the sternum (1) exposed the heart (2). After permeabilization and washing steps (3, scale bar 1mm), bulbus arteriosus (BA) and atrium (A) were removed, and only theventricle (V) was directly extracted in the IVMA flowchamber (4). (B) Protein contents of cardiac myosin extracts (CME) were analyzed via SDS-PAGE stained with Coomassie. Three main protein bands were present: one at around 200 kDa (myosin heavy chain, MHC), that was also detected by a specific antibody (AB) in WB (C), one at around 21 kDa (supposedly myosin light chain 1, MLC1) and around 14 kDa (supposedly MLC2). (C) Western blot analysis of the cardiac myosin extracts revealed the presence of the regulatory actin-connected proteins tropomyosin (Tm) and troponin T (TnT) in CME. (D) Before extraction of cardiac proteins, actin, myosin and regulatory proteins are arranged in interdigitating filaments within the sarcomere (upper part). The thin actin-containing filaments consist of actin monomers arranged in a double-helical filament (lower left panel). Filamentous Tm winds around the actin filament and is connected end-to-end via the troponin-complex. Myosin heads containing the myosin light chains protrude from the thick filament. The secondary protein structure of ELC is depicted as a ribbon model, encircled in red is the 11 amino acid part missing in the laz mutant (lower right panel; displayed via PyMOL, DeLano Scientific LLC, 2008; based on sequence from 9). (E) Schematic of IVMA: When myosin is extracted into IVMA flowchambers (A,4), the dissociated dimers are immobilized on a nitrocellulose-coated surface and will bind labeled thin filaments in a rigor-like state (upper panel). When ATP and Ca2+ are present, actomyosin cross-bridge cycling will lead to sliding of actin filaments (lower panel). |