- Title
-
Mutant Human FUS Is Ubiquitously Mislocalized and Generates Persistent Stress Granules in Primary Cultured Transgenic Zebrafish Cells
- Authors
- Acosta, J.R., Goldsbury, C., Winnick, C., Badrock, A.P., Fraser, S.T., Laird, A.S., Hall, T.E., Don, E.K., Fifita, J.A., Blair, I.P., Nicholson, G.A., Cole, N.J.
- Source
- Full text @ PLoS One
|
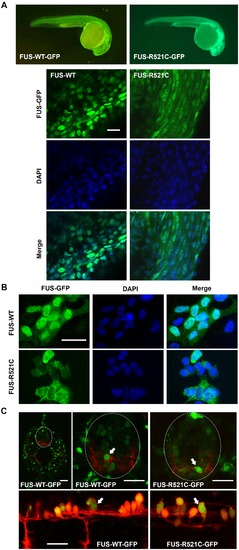
Whole mount and cell cultures of FUS-GFP transgenic zebrafish. (A) Transgenic zebrafish larvae whole mounts showed cytosolic mislocalization of mutant human FUS in FUS-R521C-GFP in comparison to FUS-WT-GFP which was restricted to cell nuclei. (B) FUS-R521C-GFP showed greater cytosolic distribution in comparison to FUS-WT-GFP in zebrafish primary cell cultures. (C) Confocal images of 48 hpf transgenic zebrafish spinal cord further demonstrate mislocalization of mutant FUS-521C-GFP (green) in motor neurons (red) (arrows). Images are maximum projections captured using a Leica SPE5 confocal microscope. Sagittal sections (upper images) of Tg(s1020tGAL4: UASmCherry) (Scott and Baier, 2009) and transverse sections (lower images) of Tg(HB9: mK02caax) membrane localised mk02 expressed in motorneurons by HB9 promoter (Flanagan-Steet et al 2005) with either FUS-WT-GFP or FUS-521C-GFP as indicated. Scale bar = 20 μm. |
|
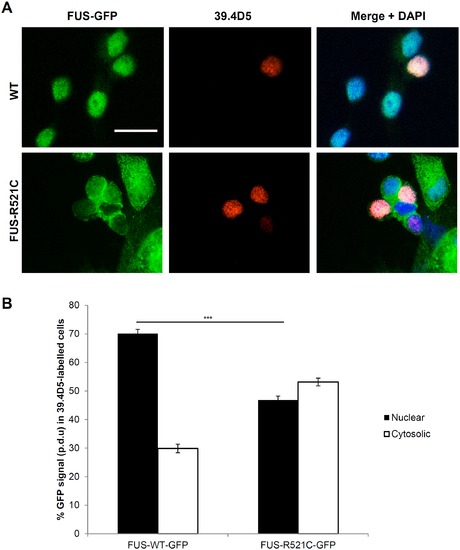
Mislocalization of mutant FUS-GFP was also found in motor neurons. (A) FUS-R521C-GFP was similarly mislocalized to the cytosol in motor neurons (labeled with 39.4D5 for islet1 and islet2 homeodomain marker). (B) Quantification of FUS-GFP signal in nucleus vs. cytosol in 39.4D5 marked motor neurons demonstrated that FUS-R521C-GFP was significantly more cytosolic compared to FUS-WT-GFP (post-hoc Tukey HSD. ** = P<0.01, N.S. = >0.05, n = 40 for all samples. Error bars represent SE. Scale bars = 20 μm. |
|
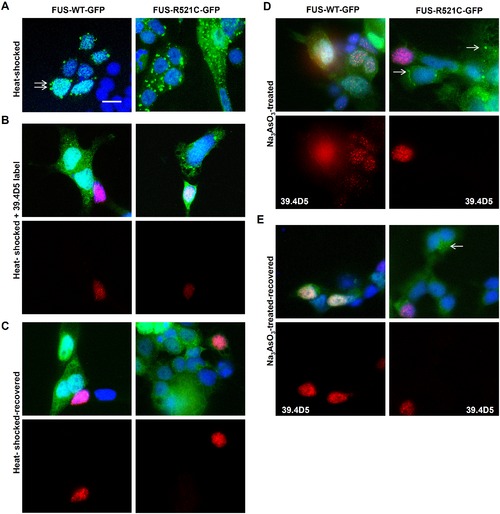
Ubiquitous FUS-GFP SG assembly in zebrafish cells. (A) FUS-GFP SGs formed in cultured transgenic zebrafish cells after heat-shock at 40°C for 30 mins (arrows). However, SGs were more abundant in mutant FUS-R521C-GFP cultures (right panels) than in FUS-WT-GFP. (B) Motor neurons (39.4D5-labeled cells) were not particularly susceptible to SG assembly. (C) SGs were reversible when cells were allowed to recover at 37°C for another 30 mins. Some persistent SGs were still present particularly in FUS-R521C-GFP cultures. Motor neurons labeled with 39.4D5 readily reversed SGs. (D) Stress granules (SGs) were also induced by sodium arsenite (Na3AsO3; 0.2 mM) treatment. SGs formed in mutant (arrows) but not in the FUS-WT-GFP line after Na3AsO3 treatment for 1 hr. Similar to heat-shocked cells, 39.4D5-labeled cells were no more susceptible to chemical-induced SG formation. (E) Chemical-induced FUS-GFP containing SGs were reversible in both lines. Reversibility also occurred readily in 39.4D5 labelled motor neurons. |
|
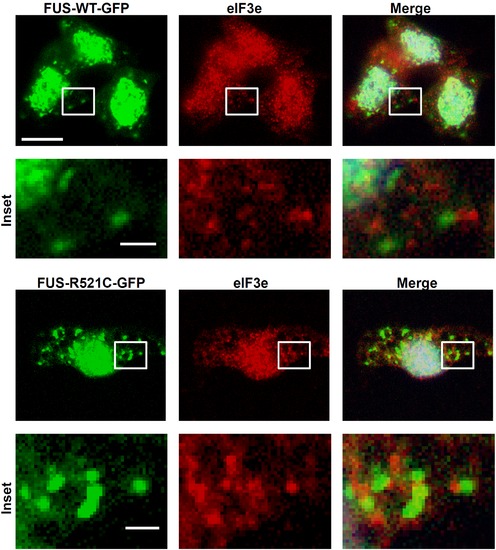
Punctuate staining with SG marker eIF3e was commonly found adjacent to or surrounding FUS-GFP SGs. Scale bars = 10 µm; Insets = 1 μm. |
|
Zebrafish cell culturing protocol supports the growth and differentiation of motor neurons. (A) Cell cultures from transgenic zebrafish embryos expressing GFP under the motor neuron promoter islet-1 (islet1: GFP) demonstrated that motor neurons represented <10% of the cells in culture and exhibited extensive differentiation with axonal growth and branching (arrow). (B) Zebrafish neural cell-associated antibodies obtained from the Developmental Studies Hybridoma Bank (University of Iowa) were used: 39.4D5 [anti-islet-1/2] ? primary motor neuron-specific transcription factor; Zn12 [anti-L2/HNK-1] ? neural cell adhesion molecule (labels many different neural subtypes); 3A10 [anti-neurofilament] - derived from a neurofilament-associated antigen and labels a subset of hindbrain spinal cord projecting neurons such as Mauthner neurons (Brand et al. 1996) but appears not to label islet 1/2 expressing motor neurons; Zn8 [anti-neurolin] - expressed by secondary but not primary motoneurons during zebrafish development. This labeling demonstrated a mix of different neural subtypes in the cultures. (C) FUS-GFP was expressed in the cell soma of motor neurons and was not extensively transported into neurites. Scale bar = 20 μm. |
|
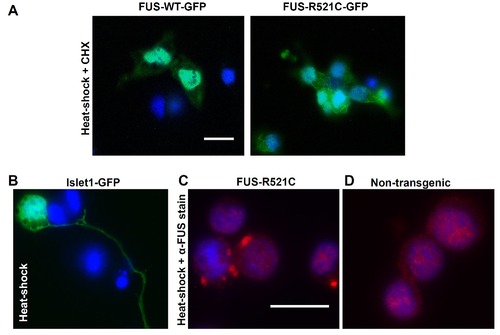
Images confirming the presence of SGs. (A) Heat-shocked cells treated prior with the SG inhibitor cycloheximide (CHX) did not form FUS-GFP containing SGs. (B) Islet1: GFP cells did not form SGs after heat-shock, indicating that the GFP tag was not involved in SG formation. (C) Non-GFP fused cultures of FUS-R521C counterstained with anti-human FUS show the presence of aggregates of similar appearance to SGs tagged with GFP. (D) The majority of non-transgenic cells did not form SGs that could be stained with anti-FUS. Scale bars = 10 μm. |
|
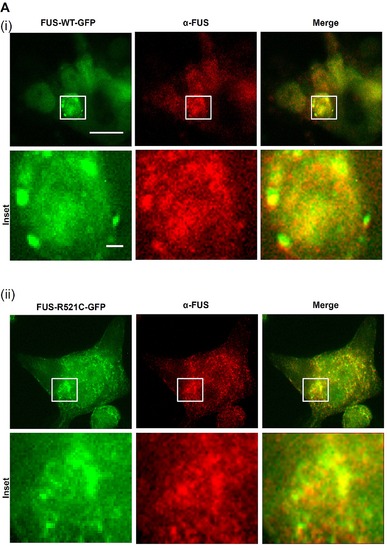
Co-localization of FUS-GFP SGs and FUS (stained with polyclonal anti-FUS antibody, red) confirms the presence of human FUS in SGs. Scale bar = 20 μm; insets = 1 μm. Brand M, Heisenberg CP, et al. (1996) Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 1236179?190. |