- Title
-
Structural and temporal requirements of Wnt/PCP protein Vangl2 function for convergence and extension movements and facial branchiomotor neuron migration in zebrafish
- Authors
- Pan, X., Sittaramane, V., Gurung, S., and Chandrasekhar, A.
- Source
- Full text @ Mech. Dev.
|
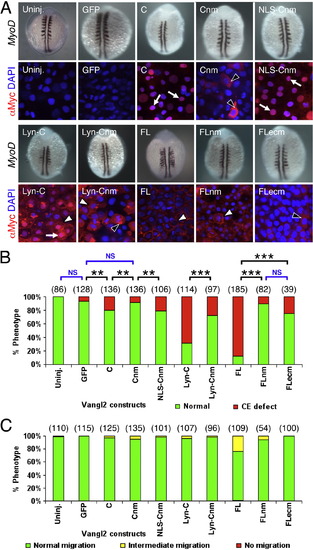
Subcellular distribution of Vangl2 variants, and differential effects of transient overexpression on CE movements and neuronal migration. (A) Two panels for each injection condition, with upper panel showing myod1 in situ of 12?14 hpf embryo, and lower panel showing anti-Myc immunostaining (red) of ectodermal cells in 10 hpf embryo, counterstained with DAPI (blue) to stain nuclei. Uninjected (Uninj.) and GFP RNA injected controls shown in leftmost panels. Following injection of Vangl2 FL RNA, the recombinant protein localizes to the plasma membrane (white arrowhead). The corresponding myod1 in situ shows that convergence and extension (CE) movements of paraxial mesoderm are reduced, resulting in thin, elongated somites. Expression of some variants generated proteins that localized largely to the cytoplasm (black arrowheads; N, PBMΔ, P2X2) or the membrane (white arrowheads; FL, TM-C, DTM) or both compartments (N-PH), while others exhibited significant nuclear localization (arrows; C, Lyn-C). N-TM overexpressing embryos showed punctate cytoplasmic localization, resembling vesicles (white arrowhead). (B) The ability of various Vangl2 constructs to generate CE defects was variable. CE movement defects were scored by examining live embryos at 12?15 hpf. Injection dose: 280 pg RNA/embryo for all constructs except Vangl2 TM-C (150 pg/embryo). Some constructs (FL, TM-C, N-PH, Lyn-C) generated CE defects in >50% of injected embryos, whereas others exhibited weaker effects (N-TM, C, DTM) or marginal effects (GFP, PBM?, N). Significant differences p < 0.05 and p < 0.001 (Pearson Chi Square test) are indicated by (**) and (***), respectively. NS, not significant. Number of embryos scored in parenthesis for each construct. Data pooled from 3 to 5 experiments. (C) Transient overexpression did not cause defects in FBM neuron migration. See Fig. 4 legend for scoring criteria. |
|
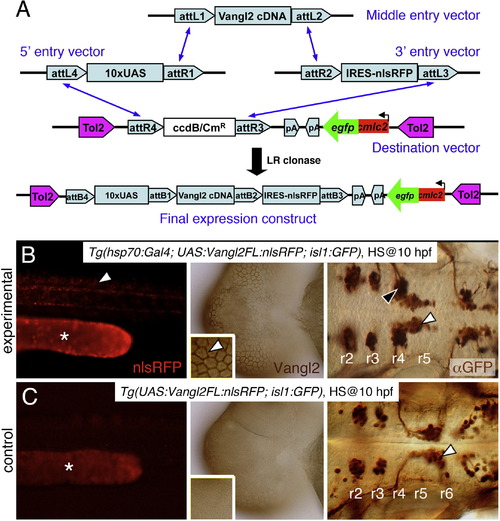
Stable heat shock-induced overexpression of Vangl2 FL causes defects in FBM neuron migration (dominant-negative phenotype). (A) Schematic outlining the Gateway cloning strategy for generating Tol2 vectors for various Vangl2 constructs used to generate stable transgenic lines. (B) In triple transgenic 48 hpf Tg(hsp70:Gal4); Tg(UAS:Vangl2FL-nlsRFP); Tg(isl1:Gfp) experimental embryos heat shocked at 10 hpf, RFP-expressing cells (left panel, white arrowhead) were distributed broadly throughout the embryo. Myc-immunostaining (middle panel, brown) showed Vangl2 protein expressed on cell membranes (inset, white arrowhead). Importantly, anti-GFP immunostaining (right panel, brown) revealed that a majority of FBM neurons failed to migrate out of r4 (black arrowhead), while several neurons had migrated into r5 (white arrowhead). (C) By contrast, in double transgenic Tg(UAS:Vangl2FL-nlsRFP); Tg(isl1:Gfp) control embryos heat shocked at 10 hpf, RFP and Vangl2-expressing cells were absent, and FBM neurons migrated normally from r4 to r6 (white arrowhead). Asterisks in RFP panels in B and C indicate autofluorescence in the yolk tube. |
|
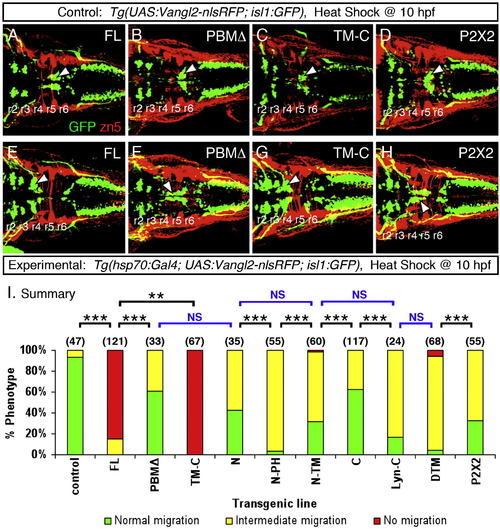
Heat shock-induced overexpression of Vangl2 FL or Vangl2 TM-C strongly interferes with FBM neuron migration (A?H). Dorsal views of 48 hpf embryos processed for anti-GFP and zn5 immunohistochemistry. The upper panels (A?D) represent the control conditions for the corresponding lower panels (E?H) representing the experimental embryos expressing different Vangl2 transgenes following heat shock at 10 hpf. In control embryos (A?D), GFP-expressing FBM neurons largely undergo normal caudal migration from r4 into r6 (arrowhead). Zn5 staining (red) labels rhombomere boundaries. The trigeminal and vagal motor neurons are located in r2 and r3, and the caudal hindbrain, respectively. In experimental embryos, Vangl2 FL and Vangl2 TM-C overexpression result in failure of caudal neuronal migration (E, G), whereas a majority of FBM neurons migrates out of r4 following Vangl2 PBM? and P2X2 overexpression (F, H). However, many neurons fail to migrate into r6, a phenotype classified as intermediate migration (see panel I). Positions of the trigeminal and vagal motor neurons are not affected by these treatments. (I) Summary of effects on FBM neuron migration following heat shock-induced Vangl2 transgene expression. Number of embryos scored in parenthesis for each construct. Data pooled from 2 to 5 experiments. Some constructs (FL, TM-C) resulted in migration failure in >80% of injected embryos. FBM neurons largely migrated out of r4 for the remaining constructs, but overexpression of some (N, N-PH, N-TM, Lyn-C, DTM, P2X2) generated intermediate migration phenotypes in >50% of embryos. Significant differences p < 0.05 and p < 0.001 (Pearson Chi Square test) are indicated by (**) and (***), respectively. NS, not significant. |
|
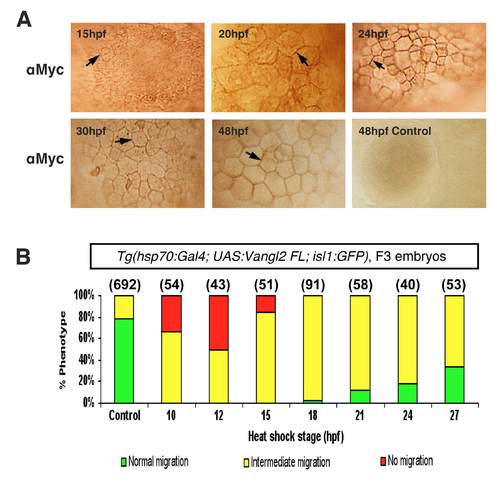
Vangl2 functions during the early stages of FBM neuron migration. Summary of effects on FBM neuron migration following heat shock-induced Vangl2 transgene expression. Embryos were heat shocked at various times (10, 12, 15, 18, 21, 24 and 27 hpf), and migration phenotypes were scored at 48 hpf. Expression of Vangl2 FL (A) and Vangl2 TM-C (B) generated similar effects. Heat shock between 10 and 15 hpf resulted in migration failure in >80% of embryos. By contrast, heat shock at 18 hpf or later resulted in few or no embryos with non-migrated neurons. However, most of these embryos exhibited varying degrees of migration block in r5 (see Fig. 4F and H). Number of embryos scored in parenthesis for each construct. Data pooled from 2 to 5 experiments. PHENOTYPE:
|
|
Potential role for nuclear localization of Vangl2 for CE movements. (A) Two panels for each injection condition, with upper panel showing myod1 in situ of 12?14 hpf embryo, and lower panel showing anti-Myc immunostaining (red) of ectodermal cells in 10 hpf embryo, counterstained with DAPI (blue) to stain nuclei. Uninjected (Uninj.) and GFP RNA injection controls are indicated. Following injection of RNA, Vangl2 C localizes strongly to the nucleus (arrows). The corresponding myod1 in situ shows that CE movements of paraxial mesoderm are not affected. Expression of Vangl2 Cnm with mutations in the putative nuclear localization motif lead to failure of nuclear accumulation, and the protein is instead found in the cytoplasm (black arrowheads). Addition of a heterologous nuclear localization signal restores Vangl2 NLS-Cnm localization to the nucleus. Whereas Vangl2 Lyn-C is frequently found in the nucleus (arrow) and plasma membrane (white arrowhead), Vangl2 Lyn-Cnm fails to localize to the nucleus, but is found in cytoplasmic puncta (black arrowhead) and on the plasma membrane (white arrowhead). (B) Mutating the nuclear localization motif affects Vangl2 activity. The ability of Vangl2 FL and Vangl2 Lyn-C to efficiently induce CE defects was essentially abolished when the putative nuclear motif was mutated. (C) Transient overexpression of Vangl2 nuclear and extracellular loop mutants did not cause defects in FBM neuron migration. See Fig. 4 legend for scoring criteria. Number of embryos scored in parenthesis for each construct. Data pooled from 3 to 5 experiments. In (B), significant differences p < 0.05 and p < 0.001 (Pearson Chi Square test) are indicated by (**) and (***), respectively. NS, not significant. |
|
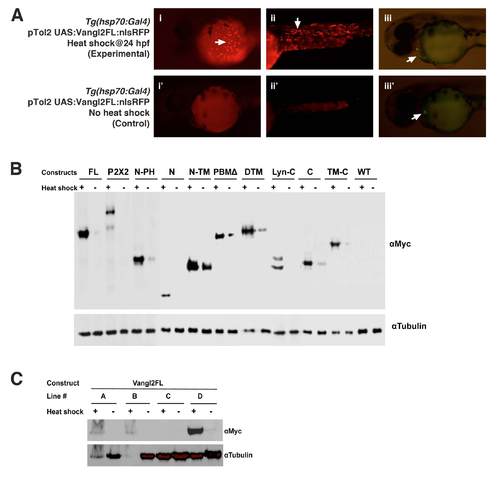
Validation of Tol2 vectors and establishment of heat shock inducible transgenic lines (A) Transient expression of Vangl2 FL protein by injecting Tol2 plasmid into Tg(hsp70:Gal4) embryos (i-iii). The 24 hpf embryos were heat shocked for 1 hr at 37°C and examined at 48 hpf. Control embryos contained the Tol2 plasmid but were not subjected to heat shock (i′-iii′). Embryos carrying the injected Tol2 plasmid express GFP in the heart (arrows in iii and iii′). Heat shocked embryos express RFP in the superficial cells of the yolk sac (i, arrow) and cells in the trunk (ii, arrow). RFP is not expressed in non-heat shocked embryos (i′, ii′). (B) Western blotting with anti-Myc antibody shows that the various Vangl2 variants are expressed in the Tol2 plasmid-injected embryos after heat shock (+). While most constructs are not activated in the absence of heat shock (-), there is a varying amount of weak (leaky) expression for N-PH, N-TM, PBM?, DTM, and C. A Western using anti-tubulin antibody was performed simultaneously to detect tubulin as a loading control. (C) Western blotting showing different levels of Vangl2 FL protein expression among four different stable transgenic lines. Lines A and D were selected for further analysis, and most experiments were performed with line D. |
|
(A) Appearance and perdurance of Vangl2 protein induced by heat shock treatment |
|
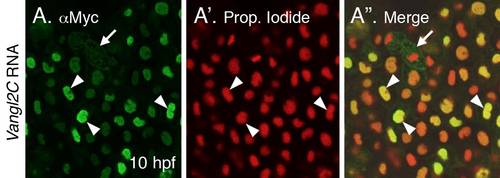
Association of Vangl2 with the nucleus (A-A′′) 10 hpf embryo injected with myc-tagged Vangl2C RNA, and processed for immunostaining with anti-Myc antibody to label Vangl2C protein (A), and counterstained with the nuclear marker propidium iodide (A′). The Vangl2C signal is essentially restricted to the nucleus (arrowheads), although some membranous and punctate staining is seen in dividing cells (arrow). |
Reprinted from Mechanisms of Development, 131, Pan, X., Sittaramane, V., Gurung, S., and Chandrasekhar, A., Structural and temporal requirements of Wnt/PCP protein Vangl2 function for convergence and extension movements and facial branchiomotor neuron migration in zebrafish, 1-14, Copyright (2014) with permission from Elsevier. Full text @ Mech. Dev.