- Title
-
New sources of retinoic acid synthesis revealed by live imaging of an Aldh1a2-GFP reporter fusion protein throughout zebrafish development
- Authors
- Pittlik, S., and Begemann, G.
- Source
- Full text @ Dev. Dyn.
|
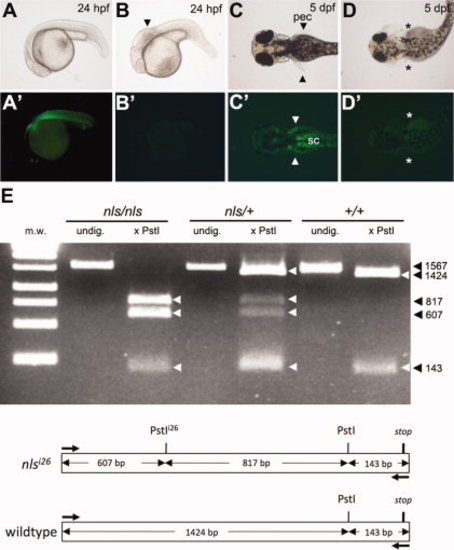
The aldh1a2:gfp transgene rescues the neckless (nls) mutant phenotype. A?D′: Offspring from an incross between nls -/-; aldh1a2-GFP +/- fish (see Table 1). All GFP-positive embryos have wild type appearance (A,C,), whereas all GFP-negative embryos (B′, D′) show the nls mutant phenotype (B,D), including a shortened hindbrain with kinked appearance (arrowhead, B) and absence of pectoral fins (asterisks, D). GFP expression in pectoral fins (pec, arrowheads) and spinal cord (sc) motorneurons at pectoral level (C′) contrasts with autofluorescence of the yolk in all embryos at 5 days post-fertilization (dpf) (C′,D′). E: PstI-digestion of a 1,567-bp endogenous aldh1a2 PCR fragment amplified from cDNA detects a restriction fragment polymorphism in the presence of the mutant nlsi26 allele. cDNA was prepared from regenerating caudal fin blastema of adult aldh1a2-GFP fish predicted to be homozygous, heterozygous, or wt for the nlsi26 allele (Table 1). Schematic shows restriction map of the amplified region. m.w., molecular weight ladder; undig., undigested. |
|
Comparison of aldh1a2 gene expression and aldh1a2:gfp reporter fluorescence. Whole mount in situ hybridization patterns of aldh1a2 gene expression resemble those of aldh1a2:gfp fluorescence. Shifting patterns of gene expression can be observed in the paraxial mesoderm (pm) (A, A′), developing somites (som) and head mesoderm (arrowhead, B, B′), dorsal retina (dr) and cells enclosing the anterior spinal cord at the level of the first 2 somites (arrow) (C, C′), branchial arch (ba) primordia, pectoral fin mesenchyme (pf) and head mesenchyme (hm) contributing to the otic region (D, D′). After hatching, additional expression domains are visible in the branchial arches (arrow), pronephric tubules (arrowhead), and spinal cord motorneurons (scm) at pectoral fin level (E?F′). Higher magnification of paraxial mesodermal cells at 12 hpf shows cytoplasmic aldh1a2 expression; notochordal cells (n) are unlabeled (G). At 6 dpf, expression is apparent in the intestinal walls of the hindgut (hg) (H?H′′), as well as in cells located distal and posterior to the first 4 branchial arches (pharyngeal arches 3?6), which are gill-bearing arches (I?L). GFP fluorescence (green channel) is distinct from chondrocytes (marked in red by a col2a1:mCherry transgenic line). Nuclei were counterstained with DAPI in embryos in G, L. The swim bladder (sb) expresses aldh1a2 at 4 dpf (M, M′). aldh1a2 is upregulated in the regenerating caudal fin fold (cff), 2 days after amputation (N), whereas unamputated controls show no reporter expression (N′). All images are oriented with rostral to the left; A?B′, D, D′, G: dorsal views; C, C′, E?F′, H, H′, K, M?N′: lateral views; I, J, L: ventral views. F′, G, H′, L: aldh1a2:gfp labeled with anti-GFP antibody. Scale bars = 250 μm (A?F, I, M, N), 100 μm (J, K), 50 μm (G, H, L). bl, blastema-like cells; cb, ceratobranchial cartilage; ch, ceratohyal cartilage; hyo, hyosymplectic cartilage; pr, proctodeum/cloaca. EXPRESSION / LABELING:
|
|
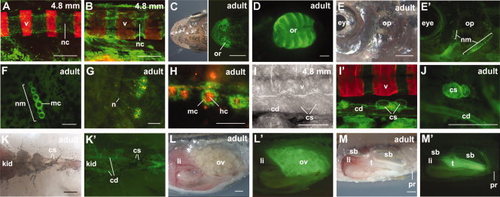
aldh1a2 expression domains in larval and adult zebrafish. Dynamic aldh1a2 expression is visible in the mineralizing vertebral column (illustrated by alizarin red staining) as a sheath covering the notochordal surface of larvae at 4.8 mm standard length at different stages of development (A, B). Stable expression was detected in the lamellae of the adult olfactory rosette (olfactory epithelium) (C, D), and in stitches of the anterior and posterior lateral line (E, E′, postoptic region and opercle). A vertical row of six neuromasts located on the dorsal head (F), in which aldh1a2:gfp-positive mantle cells surround the 4-Di-2-ASP-labelled neuromast hair cells (H); enhanced contrast visualizes accompanying nerves (G). In 4.8-mm-sized (standard length) larvae and adults, aldh1a2-positive cells are detected in the corpuscles of stannius (cs) and the collecting ducts (I?K′), as well as in the mature ovaries (ov) and testes (te) (L?M′). All images are oriented with rostral to the left; A, B, E?I′, L?M′: lateral views; C, D: dorsal views; K, K′: ventral views. Scale bars = 100 μm (A, B, D, F?I,), 1 mm (C, E, J?M). cd, collecting duct; li, liver; mc, mantle cells; n, nerves; nc, notochord; nm, neuromasts; op, opercle; or, olfactory rosette; pr, proctodeum/cloaca; sb, swim bladder; v, vertebrae. Fluorescent images of brightfield counterparts are labeled with inverted commas. |