- Title
-
Characterization of vascular mural cells during zebrafish development
- Authors
- Santoro, M.M., Pesce, G., and Stainier, D.Y.
- Source
- Full text @ Mech. Dev.
|
Histological analyses of the dorsal aorta and posterior cardinal vein of 3 months old zebrafish. (a?d) Toluidine blue-stained transverse sections of the trunk vasculature showing the dorsal aorta (DA) and posterior cardinal vein (PCV). A thick layer (arrows in (b and c)) of vascular SMCs (vaSMCs) is present around the DA but not the PCV, where single vaSMCs (arrow in (d)) are interspersed along the circumference of the PCV. Scale bars, 100 μm. *Peri-aortic melanocytes. Sections are at the level of the 10th somite. |
|
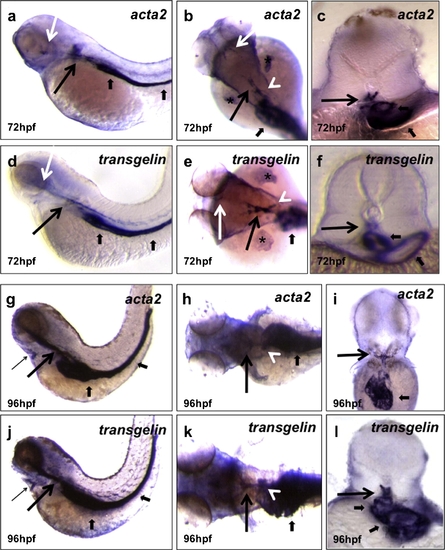
Mural cell markers are observed in specific perivascular districts. Wild-type larvae analyzed for acta2 (a?c; g-i) and transgelin (d?f; j?l) expression at 72 (a?f) and 96 (g?l) hpf. At 72 hpf, acta2 and transgelin are strongly expressed in the anterior lateral dorsal aortae (LDA) (long black arrows), internal carotid arteries (ICA) (long white arrows) and anterior mesenteric artery (AMA) (white arrowheads). At 96 hpf, a specific and diffuse staining appears at the Y junction of the bilateral dorsal aortae (long black arrows), AMA (white arrowheads) and in the heart region at the level of the bulbus arteriosus (BA) and ventral aorta (VA) (thin arrows). Visceral smooth muscle around the gut and swim bladder (short black arrows) are also evident at both stages. Cross-section analyses reveal specific staining around the dorsal aorta (vascular MCs) (long black arrows) and the gut epithelium (visceral smooth muscle cells) (short black arrows). Images (a, d, g and j) are lateral views, anterior to the left; (b, e, h and k) are dorsal views, anterior to the left; (c, f, i and l) are cross-sections at the level of the 2nd somite. At 72 hpf, both genes are also expressed in the fin buds (asterisks). EXPRESSION / LABELING:
|
|
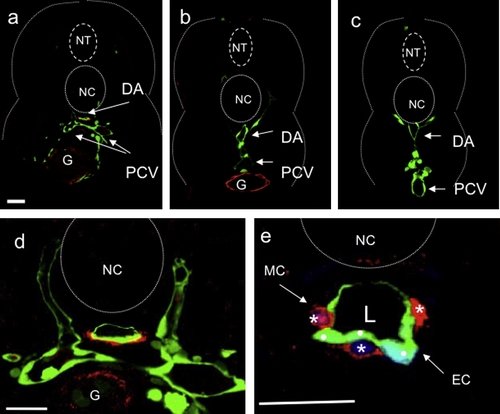
Vascular mural cells are present around the dorsal aorta in larvae. Confocal images of transverse sections of 80 hpf Tg(flk1:gfp)s843 wild-type larvae stained for Transgelin (red; (a?e)) and DNA (blue; (e)). Vascular MCs are Transgelin-positive cells surrounding endothelial cells (ECs) (green) of the dorsal aorta (DA). Sections shown are at the level of the 2nd (a, d and e), 10th (b) and 18th (c) somite. Vascular Transgelin-positive MCs are absent from the PCV in all sections (a?d). High-magnification images show that Transgelin-positive MCs (white asterisks) are single cells clearly distinguishable from endothelial cells (ECs: white dots). Scale bars, 20 μm. NT, neural tube; NC, notochord; DA, dorsal aorta; PCV, posterior cardinal vein; G, gut; L, vascular lumen. EXPRESSION / LABELING:
|
|
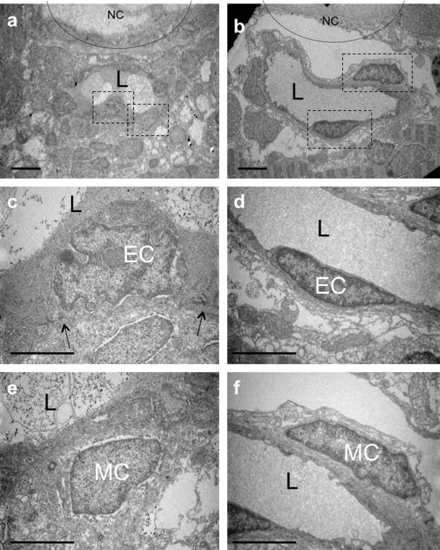
TEM of single mural cells surrounding endothelial vessels in larvae. Transmission electron microscopy (TEM) images of transverse sections of 80 (a, c and e) and 120 (b, d and f) hpf larvae. (c and e) Higher magnification of MCs and ECs boxed in (a). MCs and ECs exhibit different morphologies, and tight junctions are evident between ECs (black arrows). (d?f) Higher magnification images of MCs and ECs boxed in (b). Both ECs and MCs exhibit a more elongated morphology at 120 than 80 hpf. L, lumen. Scale bars, 5 μm. |
|
Vascular mural cells first appear in the anterior region of the dorsal aorta. (a?c) Confocal images of horizontal sections of a 80 hpf Tg(flk1:GFP)s843 wild-type larva stained for Transgelin (red) show that MCs develop as a ?bulge? of cells at the Y junction between the lateral dorsal aortae (LDA) and the dorsal aorta (DA). Images (a?d) are ventral views, anterior to the top. (d) Schematic representation of vascular MCs (red) and ECs (green) forming the anterior trunk vasculature at 80 hpf. (e) Confocal image of a transverse section of a 80 hpf Tg(flk1:GFP)s843 wild-type larva stained for Transgelin (red). Vascular MCs are present and appear to originate at the ventral region of the DA. Scale bars, 20 μm. NT, neural tube; NC, notochord; DA, dorsal aorta; PCV, posterior cardinal vein; G, gut; L, vascular lumen. EXPRESSION / LABELING:
|
|
Vascular mural cells derive from the lateral plate mesoderm but not from the blood or endothelial lineages. Confocal images of transverse sections, at different magnifications, of 80 hpf Tg(flk1:GFP)s843 wild-type (a and d) or cloche (clos5) (b and e) or hands-off (hans6) (c and f) mutant larvae analyzed for Transgelin expression (red). While wild-type (wt) and clos5 mutant larvae show Transgelin-positive cells around both the dorsal aorta and visceral organs, hans6 mutant larvae completely lack both vascular and visceral MCs. Sections are at the level of the1st-2nd somite. Scale bars, 20 μm. NT, neural tube; DA, dorsal aorta; SB, swim bladder; G, gut; MA, mesenteric artery. EXPRESSION / LABELING:
PHENOTYPE:
|
|
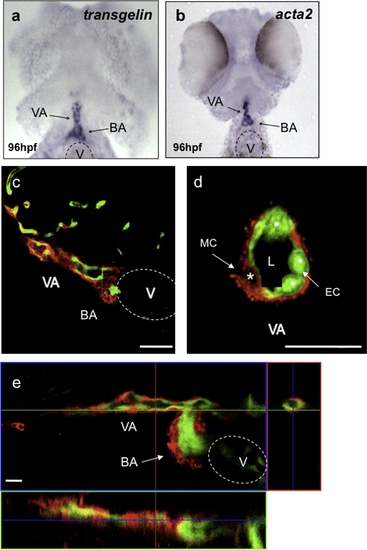
Vascular mural cells contribute to the ventral aorta and the bulbus arteriosus. Ninety-six hpf wild-type larvae analyzed for transgelin (a) and acta2 (b) expression. transgelin and acta2 are expressed in the bulbus arteriosus (BA) and ventral aorta (VA). (c) Confocal image of a sagittal section of a 96 hpf Tg(flk1:EGFP)s843 wild-type larva stained for Transgelin (red) show that vascular MCs surround the VA and contribute to the BA. (d) Confocal image of a transverse section of the VA shows single MCs around the ECs (white dots). (e) Confocal projection of the heart region of a 120 hpf Tg(flk1:EGFP)s843 wild-type larva stained for Transgelin (red). At this stage of development, vascular MCs are present in the BA as well as VA. Scale bar, 20 μm. V, ventricle; L, vascular lumen. |
|
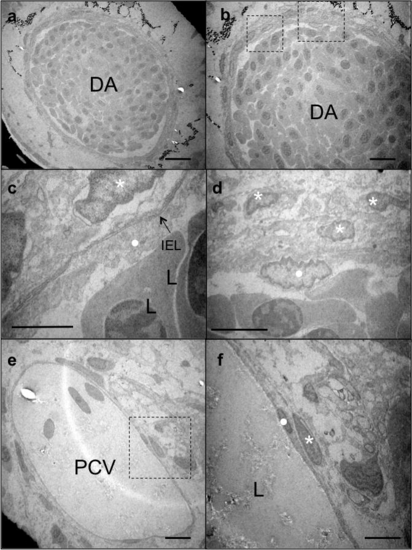
Transmission electron microscopy (TEM) images of 20 dpf zebrafish. (a and b) Sections of the DA at different magnifications, (c and d) higher magnification images of the area boxed in (b). (c) IEL is evident between EC layer (dot) and MCs (asterisk); (d) three layers of MCs are present around the EC layer, (e and f) section of the posterior cardinal vein (PCV) at high magnification, (f) higher magnification of EC (dot) and MC (asterisk) boxed in (e). Only a single layer of MCs is evident around the PCV. L, lumen; IEL, internal elastic lamina. Scale bars: 10 μm. |
|
Mural cells in the inner optic circle of 72 hpf zebrafish larvae, (a and b) whole mount in situ hybridization with acta2 shows a specific staining in the inner optic circle (IOC) (black arrows), the major central retinal vessel that surrounds the lens, (c) immunofluorescence analysis of Tg(Flk1:GFP)s843 embryos with SM22alpha antibody (red) shows the presence and localization (white arrow) of MCs in the eye. Scale bar: 0.1 mm. EXPRESSION / LABELING:
|
|
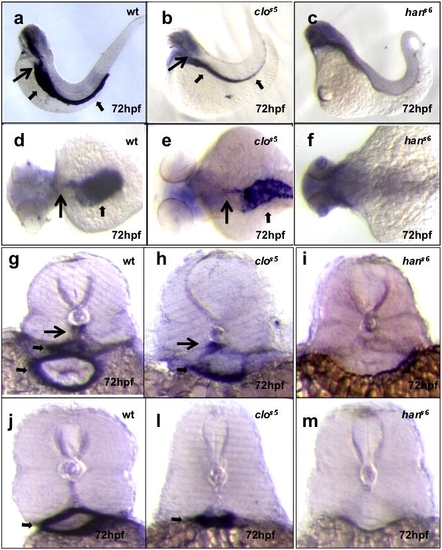
hands-off, but not cloche, mutant larvae show defective mural cell development. Seventy-two hpf larvae from wild-type crosses, or cloche (clos5) or hands-off (hans6) heterozygote incrosses analyzed for transgelin expression. In wild-type, transgelin expression identifies structures such as the dorsal aorta (long black arrows) and visceral smooth muscle (short black arrows). This expression is present in cloche mutants but missing in hands-off mutants. Images (a?c) lateral views, anterior to the left; (d?f) dorsal views, anterior to the left; (g?i) cross-sections at the level of the 1st2nd somite, and (j?m) cross-sections at the level of the 10?11th somite. EXPRESSION / LABELING:
PHENOTYPE:
|
|
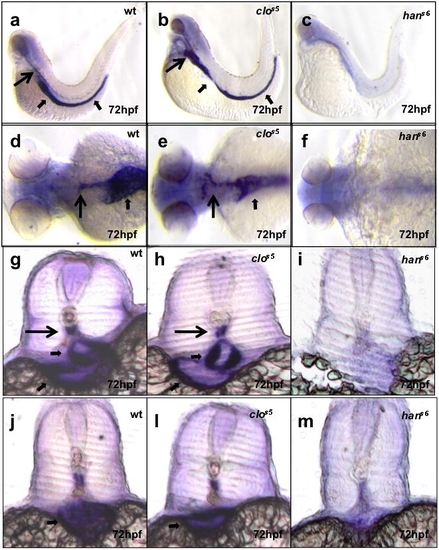
hands-off, but not cloche, mutant larvae show defective mural cell development. Seventy-two hpf larvae from wild-type crosses, or cloche (clos5)or hands-off (hans6)heterozygote incrosses analyzed for acta2 expression. In wild-type, acta2 expression identifies structures such as the dorsal aorta (long black arrows) and visceral smooth muscle (short black arrows). This expression is present in cloche mutants but missing in hands-off mutants. Images (a?c) are lateral views, anterior to the left; (d?f) dorsal views, anterior to the left; (g?i) cross-sections at the level of the 1st?2nd somite, and (j?m) cross-sections at the level of the 10?11th somite. EXPRESSION / LABELING:
|
Reprinted from Mechanisms of Development, 126(8-9), Santoro, M.M., Pesce, G., and Stainier, D.Y., Characterization of vascular mural cells during zebrafish development, 638-649, Copyright (2009) with permission from Elsevier. Full text @ Mech. Dev.