- Title
-
Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts
- Authors
- Ebert, A.M., Hume, G.L., Warren, K.S., Cook, N.P., Burns, C.G., Mohideen, M.A., Siegal, G., Yelon, D., Fishman, M.C., and Garrity, D.M.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
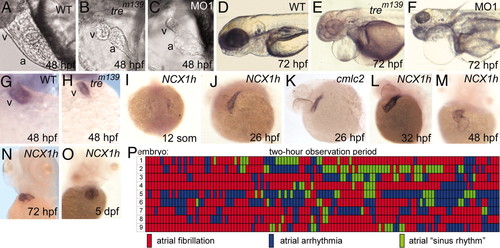
Cardiac defects in homozygous tre embryos. (A and B) Cardiac development at 48 hpf. The ventricle of tre mutants (B) is small and collapsed. (C) Injection of NCX1h-morpholino (MO1) phenocopies tre.(D?F) At 72 hpf, tre mutants and morphants show pericardial edema and slightly smaller heads. Heterozygous tre embryos (not shown) have no overt phenotype. (G and H) By 48 hpf, the ventricle, denoted by ventricle-specific vmhc expression, is markedly smaller in tre mutants. (I?O) NCX1h expression by in situ hybridization. (I) NCX1h is first detected in bilateral cardiac precursors at the 12-somite stage. (J and K) At 26 hpf, NCX1h expression in the heart tube is similar to cmlc2. NCX1h expression remains restricted to the heart at (L) 32 hpf, (M) 48 hpf, (N) 72 hpf, and (O) 5 days postfertilization. A second zebrafish NCX1 gene (NCX1b) is not expressed in the heart at any stage but is expressed in brain [see Langenbacher et al. (32)]. (P) Rhythm in the atria of nine tre embryos at 48 hpf depicted graphically. Each segment represents a 1-min observation period in which the atrial rhythm was visually scored in a single mutant embryo. Wild-type sibling embryos remained in sinus rhythm throughout the entire testing period (data not shown). No reproducible patterns in rhythm are observed on day 2 (P) or 3 (not shown) or in two other experimental replicates. a, atrium; v, ventricle. EXPRESSION / LABELING:
PHENOTYPE:
|
|
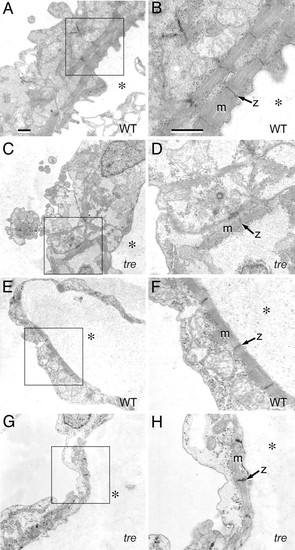
Mutation of NCX1h is associated with myofibrillar defects in the ventricle. Transmission electron micrographs of embryonic hearts at 48 hpf. (A?D) Ventricle. In the wild-type ventricle (A), myofibrillar arrays are present along the interior aspect of the chamber and (B) bundled into consecutive units. In the tre ventricle (C), sarcomeres are scarce, randomly arrayed, and form few consecutive units. (D) Myofibrillar bundles are thin and lack distinct Z lines. (E?H) Atrium. In the atrium, both wild-type (E and F) and tre mutants (G and H) assemble consecutive sarcomeric units on the interior aspect of the chamber, with no substantial defects in tre mutants. However, the atrial cytoplasmic space in tre mutants (H) is electron-lucent, potentially indicative of decreased glycogen accumulation. (Bars, 1 μm.) B, D, F, and H are magnifications of boxed regions of A, C, E, and G, respectively. Magnification in A applies to C, E, and G; magnification in B applies to D, F, and H. * denotes the interior of the heart chamber. PHENOTYPE:
|
|
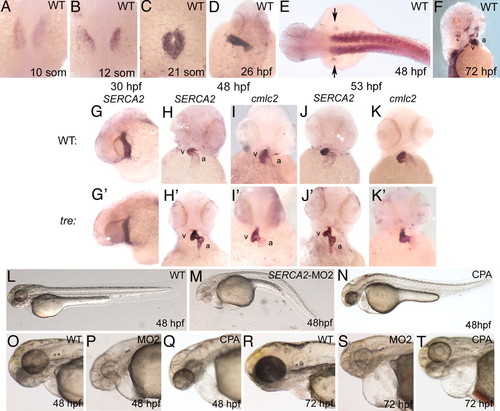
SERCA2 is essential for cardiac development. (A?F) SERCA2 expression in early development. SERCA2 RNA is expressed in (A?C) the bilateral cardiac precursors; (D) the heart tube at 26 hpf; (E) the somites and mesenchyme of the fin buds (arrows); and (F) the facial, jaw, and pectoral fin muscles at 72 hpf. (G?K2) Comparison of SERCA2 expression in wild-type (G?K) and tre mutants (G2?K2). (G and G2) At 30 hpf, SERCA2 expression in tre heart tubes is comparable to wild type. However, at (H and H2) 48 hpf, (J and J2) 53 and 72 hpf (not shown) wild-type embryos show diffuse expression of SERCA2 in the atrium, whereas tre mutants show robust expression of SERCA2 in the atrium. At (I and I2) 48 hpf and (K and K2) 53 and 72 hpf (not shown), probes for pancardiac genes cmlc2 (and tbx20, not shown) exhibit the usual diffuse signal in the atrium for both wild-type and tre embryos, indicating robust expression of SERCA2 in tre mutants is not an artifact of morphology. (L?T) SERCA2 knockdown phenotypes. Embryos injected with 17 ng of (M, P, and S) SERCA2 morpholino (MO2) or (Q and T)5 ÁM CPA have heart rates H30?50% slower than normal. Hearts show weak contractility in both chambers and fail to loop but no fibrillation or arrhythmia (other than heart rate). Most fail to circulate blood. SERCA2-inhibited embryos are touch-insensitive although not completely paralyzed. a, atrium; v, ventricle. EXPRESSION / LABELING:
PHENOTYPE:
|
|
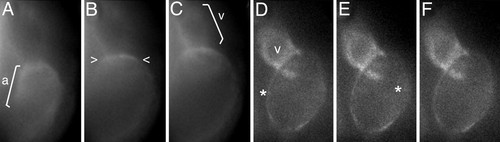
NCX1h mutation leads to calcium overload in the tre ventricle. Analysis of intracellular calcium levels in 48 hpf embryos by calcium orange fluorescent dye. Selected sequential frames at H70-msec intervals are shown. (A?D) In wild-type embryos, Ca2+ entry into cardiomyocytes, denoted by dye, sweeps across the heart in a wave (brackets) correlated with the sequential contraction of the chambers. (E?H) In tre mutants, calcium orange levels in the ventricle remain static and appear elevated relative to the atrium, suggesting calcium overload in the ventricle. Calcium signals corresponding to one or a few adjoining cells briefly fluoresce in the fibrillating atrium (see faint signals to right of *). Image is anterior to the top. a, atrium; v, ventricle. PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
|