- Title
-
Neuropilin-1a is involved in trunk motor axon outgrowth in embryonic zebrafish
- Authors
- Feldner, J., Becker, T., Goishi, K., Schweitzer, J., Lee, P., Schachner, M., Klagsbrun, M., and Becker, C.G.
- Source
- Full text @ Dev. Dyn.
|
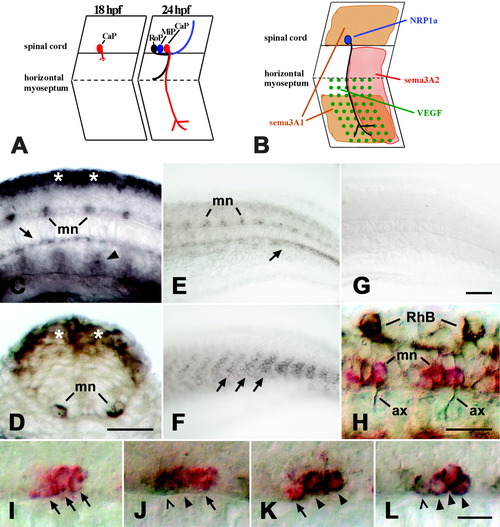
Expression of neuropilin-1a (NRP1a) and semaphorin 3A2 (sema3A2) in the trunk of embryonic zebrafish. A: A schematic side view of trunk segments at 18 and 24 hours postfertilization (hpf) is given. At 18 hpf, the caudal primary motor neuron (CaP) grows an axon out of the spinal cord. At 24 hpf, the axons of the middle (MiP) and rostral (RoP) primary motor neurons have followed on the common pathway to the horizontal myoseptum. The CaP axon is the only one growing ventrally beyond the horizontal myoseptum. B: Expression patterns of NRP1a (blue) and its potential ligands sema3A1 (orange), sema3A2 (red), and VEGF (green) are summarized for CaP in one trunk hemisegment. C: In a lateral view of a whole-mounted 16 hpf embryo at mid-trunk level (rostral is left), NRP1a mRNA is expressed in the dorsal spinal cord (asterisks), in motor neurons (mn), and in the hypochord (arrow). Arrowhead indicates expression in putative angioblasts. D: In a cross-section through the trunk at 16 hpf, expression is obvious in the dorsal spinal cord (asterisks) and the motor neurons (mn). E: In a lateral view of the caudal trunk of a 24 hpf embryo, expression of NRP1a is reduced in the dorsal spinal cord but is still strong in motor neurons (mn) and in a forming blood vessel (arrow). F: Sema3A2 is expressed in the caudal half of trunk myotomes (arrows; age and orientation as in E). G: In situ hybridization with an NRP1a sense RNA probe did not yield a signal (age and orientation as in E). H: A lateral view of a whole-mounted 24 hpf embryo is shown at caudal trunk level. NRP1a mRNA is labeled in red and tubulin protein in brown. Anti-tubulin-immunopositive motor axons (ax) can be seen to emerge from NRP1a mRNA-labeled motor neurons (mn). Rohon-Beard neurons (RhB) are labeled by the anti-tubulin antibody but not by the NRP1a in situ hybridization probe. I-L: Double labeling of NRP1a (red) and islet-1/-2 (brown) mRNAs at 24 hpf is shown (lateral trunk views, rostral is left). In I, the islet probes were omitted. In J, the NRP1a probe was combined with the islet-1 probe and in K with the islet-2 probe. In L, the NRP1a probe was combined with both probes. Arrows indicate cells labeled in red only (NRP1a). Arrowheads indicate double-labeled cells, and open arrowheads indicate cells labeled only in brown (islets). Scale bars = 25 μm in C,D,H, 50 μm in G (applies to E-G), 12.5 μm in L (applies to I-L). EXPRESSION / LABELING:
|
|
Neuropilin-1a (NRP1a) morpholinos induce aberrations of primary motor nerves. A-F: Lateral views of anti-tubulin-labeled whole-mounted 24 hpf embryos at mid-trunk levels are shown (rostral is left). A,B: In uninjected embryos (A) or those injected with 1 mM NRP1a 4mm morpholino (4mm, B), single unbranched motor nerves (arrows in A,B) grow ventrally out of the spinal cord. C-E: Injection of 1 mM NRP1a morpholino1 induced branching (arrows in C), a second exit point for motor axons per hemisegment (arrows in D), or anti-tubulin-labeled cells that appear to have migrated out of the spinal cord along the pathway of the ventral motor nerve (arrows in E). F: Injection of 1 mM NRP1a morpholino2 also induced aberrant branching (arrow) of the ventral motor nerve. Scale bar = 25 μm in F (applies to A-F). |
|
Overexpression of neuropilin-1a (NRP1a) mRNA. A,B: In lateral views of 16 hours postfertilization (hpf) whole-mount embryos (rostral is left, dorsal is up; yolk sac has been removed), myc-tagged NRP1a mRNA is expressed in a mosaic pattern (A). B: No signal is observed in uninjected animals. The insert in A shows that outlines of cells are most prominently labeled, suggesting membrane-associated expression of the exogenous protein. Scale bars = 250 μm in B (applies to A,B), 10 μm in inset. |
|
Trunk structures appear normal after injection of 1 mM neuropilin-1a (NRP1a) morpholino1. Lateral views of whole-mounted 24 hours postfertilization (hpf) embryos at mid-trunk levels are shown (rostral is left). A-F: Notochord (nc) and spinal floor plate (fp) labeled with an anti-chondroitin sulfate antibody (A,B), vertical myosepta (arrowheads) labeled with an anti-tenascin-C antibody (red), and ventral motor axons labeled with an anti-HNK-1 antibody (green, C,D), as well as muscle pioneer cells (mp) at the horizontal myoseptum labeled with an antibody to engrailed, and ventral motor axons labeled with an anti-tubulin antibody (E,F) did not show systematic differences between uninjected embryos (A,C,E) and those injected with 1 mM NRP1a morpholino1 (B,D,F). Arrows in D and F indicate aberrant branches of ventral motor nerves. Scale bars = 25 μm in B (applies to A,B), 25 μm in D (applies to C,D), 25 μm in F (applies to E,F). |
|
Other neurons and axons in the head and spinal cord are not affected by NRP1a morpholino1. Lateral views of 24 hpf whole-mounted embryos at mid-trunk (A,B,E,F) or head (C,D) levels are shown (rostral is left). A,B: Labeling Rohon-Beard (RhB) and motor neurons (mn) with an antibody to islet-1 indicates comparable numbers of these cell types in uninjected embryos (A) and those injected with 1mM NRP1a morpholino1 (B). Whereas Rohon-Beard neurons and most motor neurons are found in their correct locations, one immunopositive cell (arrow) is displaced ventral to the spinal cord in B. C,D: Anti-tubulin labeling of the dorsoventral diencephalic tract (dvdt) and the posterior commissure (pc) reveals no significant differences between uninjected embryos (C) and those injected with 1 mM NRP1a morpholino1 (D). E,F: Labeling of commissural primary ascending interneurons in the spinal cord with the 3A10 antibody indicated normal positioning of somata (CoPA) and contralateral axons (arrowheads), which eventually join the dorsal longitudinal fascicle (DLF) in uninjected embryos (E) and those injected with 1mM NRP1a morpholino1 (F). Scale bars = 25 μm in B (applies to A,B), 25 μm in D (applies to C,D), 25 μm in F (applies to E,F). |
|
Differential effects of different morpholinos on blood vessel and on motor axon development in the trunk of 24 hours postfertilization (hpf) embryos. A,C,E: Lateral views of whole embryos subjected to microangiography are shown. Fluorescence of yolk sacs is near the injection site and does not indicate the presence of blood vessels. B,D,F: Mid-trunk levels of embryos labeled with anti-tubulin antibodies subsequent to micro-angiography are shown (rostral is left). A,B: In uninjected embryos, the trunk vasculature (A, arrow) and ventral motor nerves (B) develop normally. C,D: In embryos injected with 1 mM vascular endothelial growth factor (VEGF), morpholino trunk vessels fail to develop (C) but motor nerves grow normally (D). E,F: In embryos injected with 1 mM NRP1a morpholino1, no trunk vessels are labeled (E) and ventral motor nerves grow abnormally (F). Arrow in F indicates a branched ventral motor nerve. Scale bars = 250 μm in E (applies to A,C,E), 25 μm in F (applies to B,D,F). |
|
Subthreshold concentrations of neuropilin-1a (NRP1a) morpholino1 in combination with vascular endothelial growth factor (VEGF), semaphorin (sema) 3A1, or sema3A2 morpholinos induce aberrant growth of motor nerves. Lateral views of anti-tubulin-labeled whole-mounted 24 hours postfertilization (hpf) embryos at mid-trunk levels are shown (rostral is left). A-E: Combinations of NRP1a morpholino1 with VEGF (A) or sema3A2 (D) morpholinos induce aberrant motor nerve branching (arrows in A,D), whereas displaced neurons in the path of the ventral motor nerve (arrows in B,C,E) occur after combination of NRP1a morpholino1 with VEGF (B), sema3A1 (C), or sema3A2 (E) morpholinos. Scale bar = 25 μm in E (applies to A-E). |