- Title
-
The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination
- Authors
- Wei, X., Cheng, Y., Luo, Y., Shi, X., Nelson, S., and Hyde, D.R.
- Source
- Full text @ Dev. Biol.
|
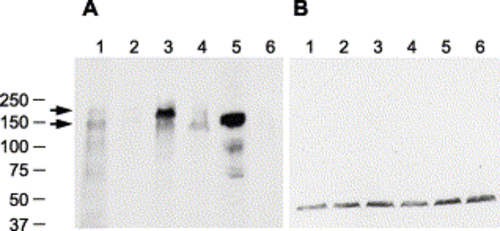
Immunoblot of Pard3 proteins. Immunoblots of protein extracts from 27 hpf embryos that were incubated with either the anti-Pard3 polyclonal antiserum (A) or anti-γ-tubulin monoclonal antibody (B), which was used as a control to confirm that equivalent amounts of protein were loaded on each lane. The lanes correspond to wild-type embryos (lane 1), embryos injected with anti-pard3 Morph-1 (lane 2), embryos co-injected with Morph-1 and mRNA encoding the 180-kDa Pard3 isoform (lane 3), embryos co-injected with Morph-1 and mRNA encoding the 150-kDa Pard3 isoform (lane 4), embryos co-injected with Morph-1 and mRNA encoding the 150-kDa Pard3 isoform fused to a six-repeat myc-epitope (which increases the protein by approximately 9 kDa, lane 5), and embryos co-injected with Morph-1 and a mRNA encoding GFP (lane 6). All mRNAs contain the β-globin 5′ and 3′ UTRs flanking the open reading frame and were transcribed in vitro. The 180- and 150-kDa Pard3 proteins are marked with arrows. The location of the BioRad molecular weight markers (in kDa) is shown to the left. EXPRESSION / LABELING:
|
|
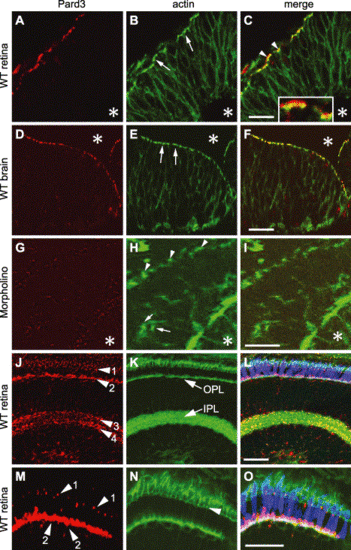
Pard3 expression in the retina and the brain. Immunohistochemistry of wild-type retinal neuroepithelium at 33 hpf (A?C), wild-type brain neuroepithelium at 33 hpf (D?F), retinal neuroepithelium of an anti-pard3 morpholino-treated embryo at 33 hpf (G?I), and a wild-type laminated retina at 5 dpf (J?O) that was stained with either anti-Pard3 antiserum (left column) or Alexa 488-conjugated phalloidin (middle column). The corresponding merged images are shown in the right column. The Pard3 protein localized to the apical region of the retinal and brain neuroepithelia at 33 hpf (A and D, respectively) and partially overlapped the adherens junction-associated actin bundles (B and E, arrows). Some of Pard3 localized slightly more apically to the actin bundles (C, arrowheads and inset). Asterisks indicate the position of the lens (A?C and G?I) or the brain ventricles (D?F). Anti-pard3 morpholinos eliminated the expression of Pard3 in the apical regions (arrowheads) of the retinal epithelium (G and I) and resulted in some mislocalization of the actin bundles (H, arrows). Pard3 localized to three major areas in the retina at 5 dpf (J?O): near or apical to the outer limiting membrane (Panels J and M, arrowhead 1), the outer plexiform layer, which correlates with the synaptic termini of the photoreceptor cells (Panels J and M, arrowhead 2), and two layers in the inner plexiform layer (Panel J, arrowheads 3 and 4). The green?red double cones were localized with the zpr-1 monoclonal antibody (L and O, blue). The section was orientated with the RPE on the top and the lens at the bottom. OPL, outer plexiform layer; IPL, inner plexiform layer. Scale bars indicate 20 μm. |
|
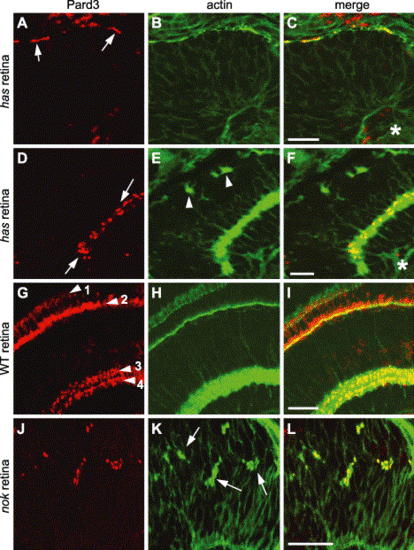
Pard3 immunolocalization in has and nok mutant retinas. Immunohistochemistry was performed on 33 hpf (A?C) and 4 dpf (D?F) hasm567 mutant retinas, 4 dpf wild-type retinas (G?I), and 33 hpf nokm520 mutant retinas (J?L) with anti-Pard3 antiserum (left column) and Alexa-488-conjugated phalloidin (middle column). The merged images are shown in the right column. Pard3 (A, arrows) localized to the apical region in the hasm567 retinal neuroepithelium at 33 hpf and partially overlapped with adherens junction-associated actin bundles. At 4 dpf, Pard3 and actin were both localized in the inner plexiform layer in the hasm567 retina (D, arrows), while actin staining in the outer plexiform layer was interrupted (E, arrowheads). In the 4 dpf wild-type retina, Pard3 and actin localized to the outer limiting membrane (arrowhead 1), the outer plexiform layer (arrowhead 2), and two sublayers in the inner plexiform layer (arrowheads 3 and 4). In the nokm520 mutant retinal neuroepithelium, Pard3 localized to the ectopic adherens junction-associated actin bundles (K, arrows). Scale bars indicate 20 μm. |
|
Morpholino-mediated reduction of Pard3 expression resulted in cell death. A lateral (A) and ventral (B) view of a wild-type embryo at 24 hpf. A plastic section of the wild-type retina and brain epithelia at 24 hpf (C). A lateral (D) and ventral view (E) of an anti-pard3 morpholino-injected embryo at 24 hpf revealed an opaque area in the head region (arrow), which suggests extensive cell death. A transverse section showed fusion of the retinas and extensive cell death in the region of the ventral diencephalon, which was characterized by darkly stained pyknotic nuclei (F, arrows). Cell viability in the retinal neuroepithelium appeared normal (F, arrowhead). Wild-type and Morph-1-treated embryos at 24 hpf were incubated in acridine orange and sectioned to identify dead cells (Panels G and H, respectively). While a few acridine orange-labeled cells were identified in the wild-type head (arrow), a larger number were present in the ventral diencephalon region in the head of the Morph-1-injected embryo (arrows). A wild-type head at 24 hpf was immunostained for actin and Pard3 expression (Panels I and J, respectively). Merged confocal image (K) confirmed that Pard3 expression is present in the apical region of the neuroepithelial cells of the ventral diencephalon at the time when significant cell death is observed in Morph-1-injected embryos. The lens (L) and retina (R) are labeled in some panels. Scale bars indicate 50 μm. PHENOTYPE:
|
|
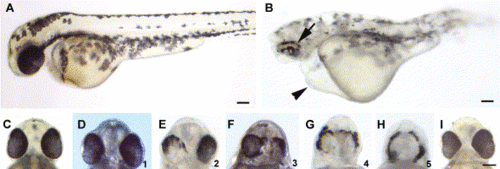
Morpholino-mediated reduction of Pard3 expression caused patchy RPE pigmentation and cyclopia. A wild-type and anti-pard3 morpholino-injected embryo at 2 dpf (A and B, respectively). The patchy pigmentation in the RPE (arrow) and the pericardial edema (arrowhead) in the morpholino-treated embryo are marked. The ventral head region of a wild-type embryo (C) shows the normal spacing between the eyes. A spectrum of cyclopic phenotypes (D?H) in anti-pard3 morpholino-treated embryos exist from the wild-type distance between the eyes (D) to a single eye (H). The numbers in the lower right corner of Panels D?H (ranging from 1 to 5, respectively) represent the degrees of cyclopia expressivity that were used to determine the cyclopia index in Table 2 and Table 3. Co-injection of in vitro-transcribed pard3 mRNA (encoding the 150-kDa Pard3 isoform flanked by the β-globin 5′ and 3′ UTRs) with the anti-pard3 morpholino (Morph-1) rescued the Morph-1-induced cyclopia phenotype and the patchy RPE pigmentation defect (I). Scale bars indicate 100 μm. PHENOTYPE:
|
|
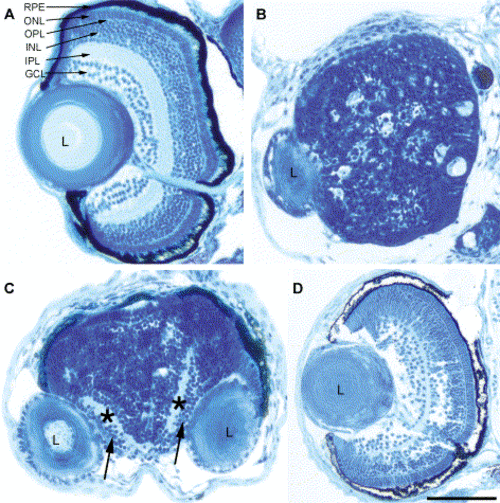
Loss of Pard3 function disrupted retinal patterning. At 5 dpf, histology was performed on a wild-type embryo (A), an anti-pard3 morpholino-injected cyclopic embryo (B), an anti-pard3 morpholino-injected embryo that developed fused retinas with separated lenses (C), and an embryo that was co-injected with anti-pard3 Morph-1 and in vitro-transcribed pard3 mRNA (encoding the 150-kDa Pard3 open reading frame flanked by the β-globin 5′ and 3′ UTRs; D). The ganglion cell and inner plexiform layers are marked with arrows and asterisks, respectively (C). RPE, retinal pigmented epithelium; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; L, lens. Scale bar indicates 100 μm. PHENOTYPE:
|
|
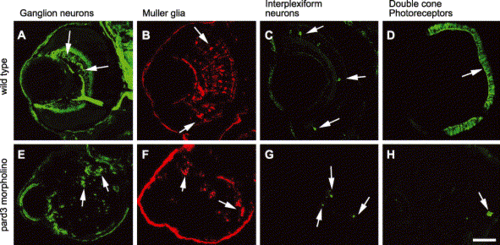
Loss of the Pard3 function did not affect the specification of retinal cells. The retinas of 5 dpf wild-type (A?D) and 5 dpf anti-pard3 morpholino-injected embryos (E?H) were immunostained with zn-8 for ganglion cells (A and E, arrows), anti-carbonic anhydrase for Müller glia (B and F, arrows), anti-tyrosine hydroxylase for interplexiform cells (C and G, arrows), and zpr-1 for double cone photoreceptors (D and H, arrows). Scale bar indicates 50 μm. PHENOTYPE:
|
|
Overexpression of Pard3 results in cyclopia. Ventral views of the head region of a wild-type embryo at 2 dpf (A), a cyclopic embryo produced by injection of anti-pard3 Morph-1 (B), and a cyclopic embryo generated by injection of wild-type pard3 mRNA (C). In all the pard3 mRNA-injected embryos, the RPE pigmentation was normal. Overexpression of the 150-kDa Pard3 isoform resulted in cyclopic embryos that had normal retinal lamination 95% of the time and disrupted lamination 5% of the time (Panels D and E, respectively). Overexpression of the pard3 mRNA encoding the 180-kDa Pard3 isoform also generated a cyclopic phenotype with 54% of the retinas possessing disrupted lamination (F). The embryos injected with pard3 mRNA also exhibited pericardial edema and curvature of the body axis (data not shown), which may represent nonspecific effects of the mRNA injections as injection of in vitro-transcribed GFP mRNA produced similar frequencies of these phenotypes as pard3-injected embryos. RPE, retinal pigmented epithelium; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars indicate 100 μm. |
Reprinted from Developmental Biology, 269(1), Wei, X., Cheng, Y., Luo, Y., Shi, X., Nelson, S., and Hyde, D.R., The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination, 286-301, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.