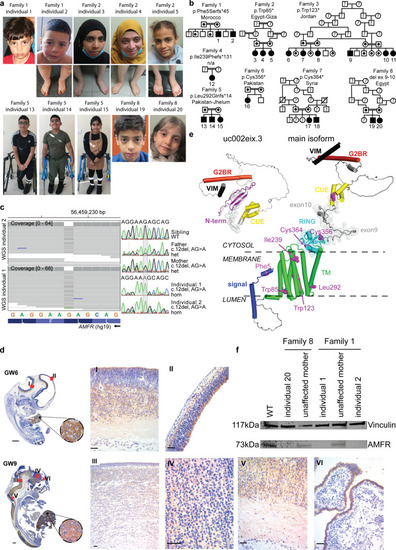
Homozygous AMFR loss-of-function variants in 20 individuals affected by HSP. a Clinical photographs of affected individuals at various ages. No major dysmorphic features are observed. b Family pedigrees of ascertained individuals, with familial AMFR variant and ethnic origin of families indicated. HSP-affected individuals with bi-allelic AMFR variants are indicated with black-filled symbols and numbered. Unaffected, heterozygous individuals are marked with a black dot. Unaffected individuals with wild-type alleles are represented by the non-filled symbols, whereas unaffected individuals of which no DNA was available for genotyping are marked with a question mark. Consanguineous parents are indicated with a double connection line. Males are squares; females are circles. c Genetic investigations in Family 1. Left panel shows aligned WGS reads from individual 1 and individual 2 in the IGV genome browser (left to right is from the centromeric to telomeric direction on chromosome 16q12.2), identifying a homozygous chr16(GRCh37):g56459228del in the first exon of AMFR. Right panel shows Sanger sequencing chromatograms (in the same orientation as the IGV genome browser view) from both affected homozygous individuals 1 and 2, both unaffected parents that are heterozygous for the deletion, and the unaffected, third oldest brother that is homozygous for the wild-type allele. Full segregation of the single-nucleotide deletion with the HSP phenotype in the Family 1 is shown in Supplementary Fig. 2. d Immunohistochemistry detecting AMFR in human fetuses at gestational week (GW) 6 and GW9. To characterize AMFR expression in various human tissues, we performed immunohistochemistry of human fetuses during the first trimester of pregnancy, using a C-terminal epitope recognizing antibody. AMFR staining of hepatocytes was noticed in all fetuses at GW6 and GW9. Central nervous system staining includes pale labeling of neuropil in the proliferative neuroepithelium of the hypothalamic, cortical, mesencephalic, and thalamic region (I, II, III and IV), as well as the marginal zone of the spinal cord (V) and cuboidal cells of choroid plexus (VI). e Structural protein model for the main AMFR isoform and the truncated isoform uc002eix.3, including the various variants identified in this study. Signal peptide is kept intact in the model to show the frameshift mutation, p.Phe5Serfs*45. The relative positions of AMFR domains are modified for clarity from the model predicted by AlphaFold2 (accession number AF-Q9UKV5-F1) [44]. Domains are color-coded. Other regions, including loops and unassigned structural elements, are shown in gray. Residues linked to patient variants are shown as pink sphere models. The region encoded by exon 9 and exon 10 is indicated by a transparent surface. The membrane and its cytosolic and luminal sides are outlined by dashed lines. Phobius (https://phobius.sbc.su.se) predictions were used to support the membrane location of AMFR regions. The main AMFR isoform contains an N-terminal signal sequence (residues 1–37), a multipass transmembrane domain (TMD) (residues 82–302), a cytosolic C-terminus which carries a Really Interesting New Gene (RING)-type zinc finger (residues 327–382), a coupling of ubiquitin conjugation to the ER degradation (CUE) domain (residues 457–497), a UBE2G2-binding region (G2BR) (residues 574–600), and a Valosin-containing protein (VCP)-interacting motif (VIM) (residues 622–640). These domains are connected by disordered regions, with residues 504–579 being compositionally biased toward polar and charged residues [43]. AMFR has an unusually open arrangement of the TMD helices, suggesting that they may form homo- or heterocomplexes. Nonetheless, the TMD appears dispensable for AMFR ligase activity [20]. The RING and G2BR domains interact with the E2 ubiquitin-conjugating enzyme UBE2G2, and the CUE domain recognizes ubiquitins on substrates. Association between the G2BR domain with UBE2G2 causes conformational alterations in UBE2G2, increasing affinity of UBE2G2 for AMFR. The VIM interacts with the AAA ATPase and segregase VCP/p97 [43]. Finally, the RING domain is tethered to the last TMD helix. Transcript uc002eix.3 (not annotated in NCBI) encodes a truncated form of AMFR (277 amino acids, 33 kDa), starting at an out of frame ATG in main transcript exon 7. This isoform lacks the TMD and RING domains N-terminal to the CUE domain, and therefore does not harbor E3 ligase activity. It has acquired 60 N-terminal residues without significant sequence identity to regions of known function for which AlphaFold2 predicts, with low confidence, the presence of a beta-sheet. f Western blotting analysis detecting AMFR and Vinculin in patient-derived fibroblasts from Family 1 and Family 8 and controls, showing the absence of the main AMFR protein isoform in patient cells. Full-length uncropped Western blot is given in Supplementary Fig. 4c
|

