|
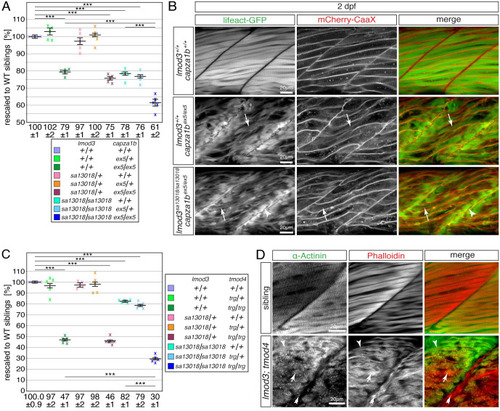
The myofibril defects of <italic toggle='yes'>tmod4</italic>-deficient mutants are distinct from the defects of <italic toggle='yes'>lmod3-</italic> and <italic toggle='yes'>capza1b</italic>-deficient mutants.(A) After rescaling to WT siblings (100 ± 1%), the significant reduction of birefringence of single lmod3sa13018 (78 ± 1%) and capza1bex5 (79 ± 1%) homozygotes was further reduced in lmod3sa13018/sa13018;capza1bex5/ex5 compound homozygotes (61 ± 2%). (B) At 4 dpf, lmod3sa13018;capza1bex5 compound homozygotes featured thin filament deposits at the peripheral ends of myofibres (arrowhead), which were also highlighted in single capza1bex5 homozygotes by Lifeact-GFP (green) and mCherry-CaaX (red). The strong myofibril striation seen in WT siblings appeared severely reduced in capza1bex5 homozygotes and was rarely detected in lmod3sa13018;capza1bex5 compound homozygotes (arrow). (C) After rescaling to WT siblings (100.0 ± 0.9%), the significant reduction of birefringence of single lmod3sa13018 (82 ± 1%) and tmod4trg homozygotes (47 ± 1%) was further reduced in lmod3sa13018;tmod4trg compound homozygotes (30 ± 1%). (D) At 3 dpf, the typical myofibril striation was highlighted by antibodies against actinin (green) and actin-labelling with phalloidin (red). In lmod3sa13018;tmod4trg compound homozygotes striation was rarely seen (arrowhead). Instead, actin- and actinin-positive aggregates were detected throughout myofibres (arrow) as well as actin-positive deposits close to vertical myosepta (double arrow). Data are presented as mean ± SEM; *** P < 0.001 calculated by one-way ANOVA with post hoc Tukey’s test; n = 5 clutches. Scale bar sizes are 20 μm.
|