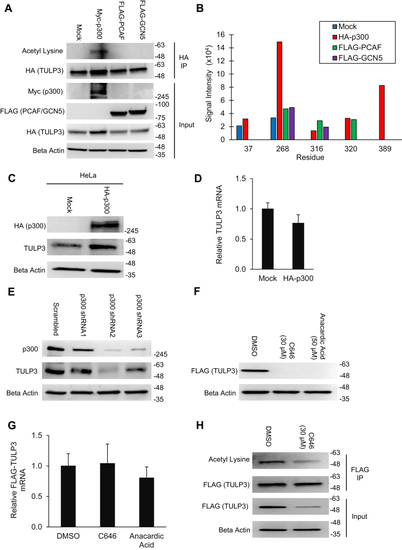
Acetylation of TULP3 by p300 increases its protein abundance in cells. A, western blot showing TULP3 acetylation levels in 293T cells in the presence of either empty pcDNA 3.1(+), Myc-p300, FLAG-PCAF, or FLAG-GCN5 following immunoprecipitation. Cells were harvested 48 h posttransfection. B, semiquantitative LC-MS/MS-based comparison of TULP3 acetylation corresponding to the conditions in (A). C, western blot showing total levels of endogenous TULP3 in HeLa cells following transfection with empty pcDNA 3.1(+) or a plasmid encoding HA-p300. Cells were harvested 48 h posttransfection. D, mRNA levels of endogenous TULP3 corresponding to samples in (C) examined using quantitative real-time PCR; n = 4 technical replicates, Mean ± S.D. shown. E, western blot of p300 and TULP3 protein levels following stable transduction of p300 shRNAs into HeLa cells. F, immunoblot of stably expressed FLAG-TULP3 protein in 293T cells following treatment with either DMSO, 30 μM C646, or 50 μM anacardic acid for 24 h. G, mRNA levels of FLAG-TULP3 corresponding to samples in (F) analyzed using quantitative real-time PCR; n = 4 technical replicates, Mean ± S.D. shown. H, acetylation levels of FLAG-TULP3 in stably expressing 293T cells following treatment with DMSO or 30 μM C646 for 9 h.
|