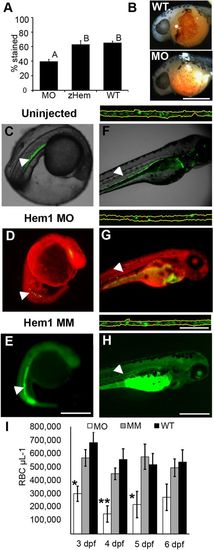
Morpholino targeting of Hemogen inhibits erythropoiesis in embryonic zebrafish. Embryos were injected with 2 to 4?ng antisense MO targeted to the first 25 coding nucleotides of Hemogen. (A-B) O-dianisidine staining of erythrocytes was decreased in morphants (MO) relative to wild-type embryos (WT) or embryos rescued with 500?pg synthetic Hemgn mRNA (zHem) at 24?hpf. (ANOVA, Tukey post hoc test, P<0.001). (C-E) Live wild-type (C), Hem1 MO-injected (D) and Hem1?mm mismatch MO-injected (E) Tg(Lcr:EGFP)cz3325Tg embryos at 20?hpf. Morphants showed decreased EGFP expression in the ICM compared to the wild-type and mismatch MO controls. (F-H) Live wild-type (F), Hem1 MO-injected (G) and Hem1?mm MO-injected (H) embryos at 72?hpf. Morphant embryos have fewer EGFP+ cells in circulation compared to the two controls. The dorsal aortas of embryos (insets above F-H) were magnified 20× to permit quantitation of EGFP+ erythrocytes. Background red (D,G) and green (E,H) fluorescence was generated by the fluorescent labels on the MOs. (I) In vivo flow quantitation of EGFP+ erythrocyte concentrations between 3 and 6?dpf in Hem1-injected (n=9,7,7,7), Hem1mm-injected (n=13,14,11,11) and uninjected (n=5,10,10,9) embryos. Data shown as meansħs.e.m. (*P?0.05, **P?0.001, ANOVA, Tukey-Kramer post hoc test). Arrowheads show notochord kinking. Scale bars: 500?µm (A-F); 100?µm (inset).
|

