Fig. 1
- ID
- ZDB-FIG-180523-18
- Publication
- Leighton et al., 2018 - An ancient conserved role for prion protein in learning and memory
- Other Figures
- All Figure Page
- Back to All Figure Page
|
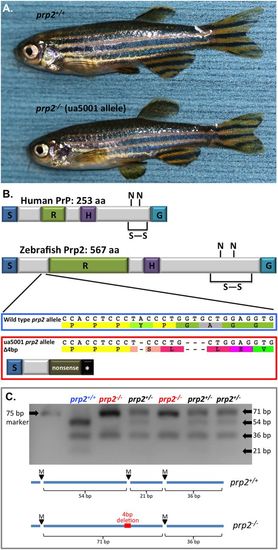
Prion mutant prp2?/? zebrafish develop normally and display no overt phenotypes during adulthood. (A) A young adult (?1-year-old) prp2+/+ fish is pictured on top, while a young adult prp2?/? fish is pictured on the bottom. (B) Zebrafish Prp2 is conserved with mammalian PrPC at the protein domain level. Both have a signal peptide (S), a repeat region (R; though the repetitive region in zebrafish is longer and less patterned than the octarepeats in mammals), a hydrophobic domain (H) and are attached to the cell surface by a GPI anchor (G). Like mammalian PrPC, zebrafish Prp2 also has putative N-linked glycosylation sites (N) and a disulphide bond (S?S) within its C-terminus. The zebrafish prp2 ua5001 allele has a 4 base pair deletion (frameshift), which produces an early stop codon and a putative nonsense protein lacking all these conserved domains. Further, the prp2 gene product is greatly reduced in abundance, in prp2?/? mutant zebrafish, including in the brain tissue of adult fish (Fleisch et al., 2013). (C) Restriction fragment length polymorphism (RFLP) assay used for genotyping zebrafish at the prp2 gene locus. There is an Mva cut site in the wild-type prp2 sequence that is not present in the mutant (ua5001 allele) prp2 sequence, leading to a smaller band when the PCR product from prp2+/+ zebrafish is digested (54?bp) compared to from prp2?/? zebrafish (71?bp). bp, base pair. |