Fig. 3
- ID
- ZDB-FIG-161118-8
- Publication
- Han et al., 2016 - ?E-catenin-dependent mechanotransduction is essential for proper convergent extension in zebrafish
- Other Figures
- All Figure Page
- Back to All Figure Page
|
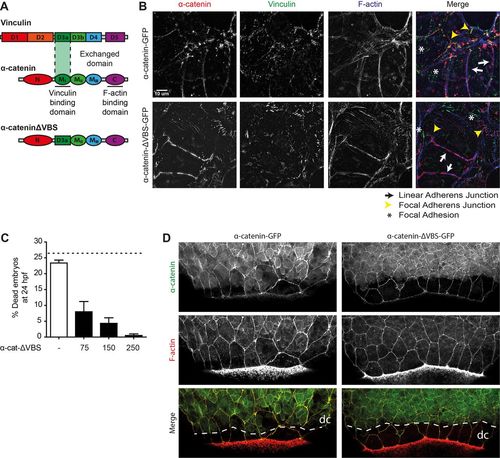
The vinculin-binding domain in ?E-catenin is not needed for barrier function. (A) Schematic representation of the structures of ?-catenin, vinculin and the exchanged domain in ?-catenin-?VBS. The domain containing the vinculin binding site (VBS) was replaced by a homologous domain from vinculin itself, which does not bind vinculin. (B) Images of fixed ?-catenin-deficient DLD1 R2/7 cells expressing zebrafish ?-catenin-GFP (top) or ?-catenin-?VBS-GFP (bottom) (both depicted in red), and stained for vinculin (green) and F-actin (blue). Linear adherens junction structures are highlighted by the white arrows, focal adherens junctions with the yellow arrowheads, and focal adhesions with white asterisks. (C) Rescue of embryo lysis in ctnna1 mutants using increasing concentrations of ?-catenin-?VBS-GFP mRNA. The dashed line indicates the expected mortality of non-injected incrossed heterozygous ctnna1 mutants based on Mendelian genetics. Data represents three independent experiments, n>120 embryos per condition. Data is represented as the meanąs.e.m. (D) Whole-mount immunostaining of zebrafish embryos at 85% epiboly expressing either ?-catenin-GFP (left) or ?-catenin-?VBS-GFP (right) (both depicted in green) and stained for F-actin (red). The dashed white line marks the deep cell margin (dc) while the actin ring marks the EVL margin. |