- Title
-
Loss of zebrafish Smyd1a interferes with myofibrillar integrity without triggering the misfolded myosin response
- Authors
- Paone, C., Rudeck, S., Etard, C., Strähle, U., Rottbauer, W., Just, S.
- Source
- Full text @ Biochem. Biophys. Res. Commun.
|
Smyd1a is specifically expressed in the heart and fast-twitch skeletal muscle cells. (A, B) smyd1a mRNA is strongly expressed in somatic muscle cells and both heart chambers (inset) as revealed by ISH. (B) Smyd1 specific RT-PCR confirms expression of all smyd1 transcription variants in embryonic heart tissue. (C) Smyd1a-specific ISH and immunostaining of slow-twitch skeletal muscle fibers (slow MHC) in a transversal section of a zebrafish embryo. (D) Fluorescent imaging of transgenic lines carrying either a smyd1b_tv1-gfp fusion construct or smyd1a-gfp fusion construct under the muscle specific unc45bmin-promoter in combination with co-immunostaining against sarcomeric ?-Actinin (Z-disc marker). EXPRESSION / LABELING:
|
|
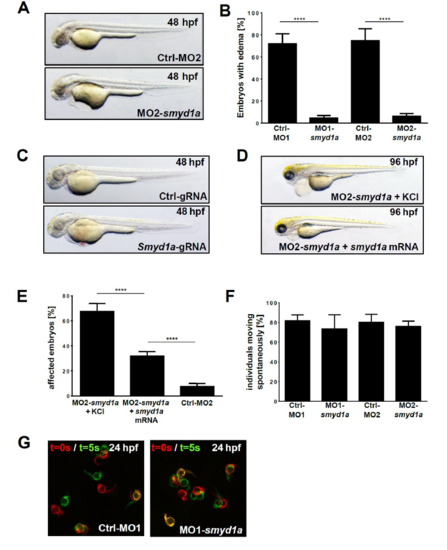
Knock-down of smyd1a leads to defective skeletal muscle and heart function. (A) Zebrafish embryos at 48 hpf injected either with an morpholino against smyd1a (MO1-smyd1a) or the respective control (Ctrl-MO1). (B) Quantification of ventricular FS in % (n = 6) from A and B at 72 hpf. Shown are mean values of three independent experiments with error bars representing standard deviation (SD). Statistical analysis was performed using one-way ANOVA followed by tukey's multiple comparison analysis (****p < .0001). (C) Quantification of the touch-evoke response assay. Smyd1a morphants were analyzed in regard to their adequate touch responsiveness upon mechanical stimulus. Shown are mean values (+/? SD) in percent of three independent experiments. Statistical analysis was performed as in (B) (C) Birefringence images of embryos (48 hpf) injected with Ctrl-MO1, MO1-smyd1a or Cas9 + smyd1a-gRNA ribonucleoprotein(RNP) complex. (E) Electron micrographs of skeletal muscle cells of smyd1a morphants versus control embryos at 48 hpf. PHENOTYPE:
|
|
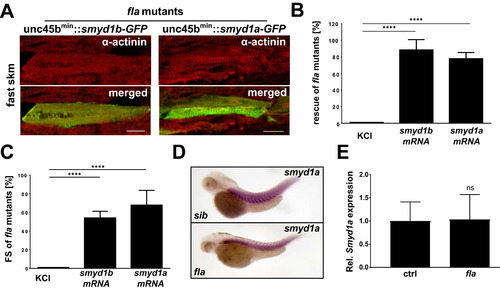
Smyd1a is required for proper sarcomere organization and can compensate for the loss of Smyd1b. (A) Co-immunostaining of plasmid (unc45bmin:smyd1b_tv1-gfp- or with unc45bmin:smyd1a-gfp) injected fla mutants with sarcomeric ?-Actinin. [Scale bar: 20??m]. (B) Flatline embryos were injected with either KCl (control), smyd1b_tv1 or smyd1a mRNA. At 72 hpf flatline mutants were identified and evaluated in regard to restored cardiac contractility. Shown are the mean percentages (+/? SD) of rescued fla embryos (n = 80) of four independent experiments. Statistical analysis was performed using one-way ANOVA (****p < .0001). (C) Measurement and statistical analysis as in (B) of ventricular FS of rescued fla mutants by injection of smyd1a- or smyd1b-mRNA at 72 hpf. Data are mean values?±?SD. (D) Smyd1a specific ISH in fla mutants compared to control siblings (sib). (E) qRT-PCR analysis of smyd1a expression in control versus fla embryos. EXPRESSION / LABELING:
|
|
Loss of Smyd1a does not trigger the misfolded myosin response. (A) ISH analysis of smyd1a expression in unc45b mutant embryos (steif) or embryos injected with a Cas9-protein/hsp90aa1-gRNA complex, respectively. (B) mRNA levels of the muscle chaperones unc45b and hsp90aa1 are strongly upregulated in muscle cells of fla mutants, whereas their expression is not affected by loss of smyd1a. (C) Rescue of fla mutants by injection of either smyd1a- or smyd1b-mRNA. EXPRESSION / LABELING:
PHENOTYPE:
|
|
(A) Lateral view of zebrafish embryos at 48 hpf injected either with an ATG-start morpholino against smyd1a (MO2-smyd1a) or a 5 bp mismatch morpholino as the respective control (Ctrl-MO2). (B) Quantification and statistical analysis of affected embryos showing pericardial edemaafter injection of the corresponding morpholinos. Statistical analysis of five independent experiments was performed using one-way ANOVA (****p<0.0001). Data are mean values +- SD. (C) For the generation of CRISPR/Cas9-mediated mutants (crispants) Cas9-Protein in complex with tracrRNA and smyd1a-targeting crRNA was injected into one-cell stage embryos and compared to a non-targeting gRNA (Ctrl-gRNA). Shown are lateral views of embryos at 48 hpf. (D) Lateral view of the rescued morphant phenotype (96 hpf) by injecting smyd1a-mRNA and MO1-smyd1a together in comparison to MO1-smyd1a + KCl injected embryos. (E) Quantification of affected embryos with heart and swim defects of smyd1a morphants after injection of MO2-smyd1a + KCl or together with smyd1a-mRNA at 96 hpf. The 5 bp mismatch morpholino (Ctrl-MO2) served as negative control. Shown are mean values of 4 independent experiments (+- SD) and statistical analysis was performed as in C (**** p<0.0001). (F) Quantification of the spontaneous movement assay of Ctrl-MO1/2 and MO1/2-smyd1a injected embryos. (G) Spontaneous movement assay with false-coloured superimposed overviews of 24 hpf control (Ctrl-MO1) injected and MO1-smyd1a injected embryos. Red: t = 0 sec; green: t = 5 sec. |
|
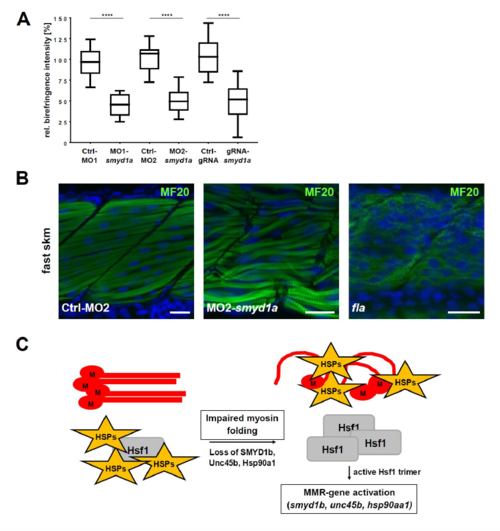
(A) Measurement of the birefringence intensity of control injected (Ctrl-MO1/-MO2 and Ctrl-gRNA) and smyd1a deficient (MO1-/MO2-smyd1a and smyd1a-gRNA) embryos. Signal intensities of n=10 embryos were normalized against the mean of the respective control measurement. Data are shown as mean value (box = 25th and 75th percentile; error bars = min/max).Statistical analysis of three independent experiments was performed using one-way ANOVA followed by tukey?s multiple comparison analysis (**** p<0.0001). (B) Myosin specific whole mount immunofluorescence stainings of control morpholino (Ctrl-MO1) injected embryos and MO1-smyd1a morphants in comparison to homozygous fla mutants which lack Smyd1b expression. In contrast to a decreased myosin protein level in fla mutants, structural disorganization of myofibers is observed in smyd1a deficient morphants. (C) Schematic overview of the misfolded myosin response (MMR) after Etard et al. (2015). PHENOTYPE:
|