- Title
-
Granule cells control recovery from classical conditioned fear responses in the zebrafish cerebellum
- Authors
- Matsuda, K., Yoshida, M., Kawakami, K., Hibi, M., Shimizu, T.
- Source
- Full text @ Sci. Rep.
|
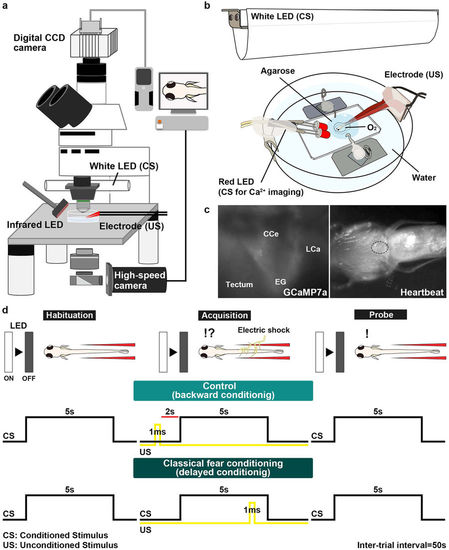
Method for investigating classical fear conditioning in zebrafish. (a,b) Schematic of the experimental setup for classical fear conditioning using zebrafish larvae at about 20 dpf. The larva was restrained in agarose gel, and O2 gas was provided from below to support respiration. Extinguishment of a white or red LED was given as the CS, and an electric shock was given as the US. (c) Imaging of Ca2+ signals of cerebellar neurons (left panel); and heartbeats (HB, right panel) in Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) larvae. Images showing the movement of the heart were obtained from underneath with an infrared digital camera. The heart is indicated by a dotted circle. CCe, corpus cerebelli; EG, eminentia granularis; LCa, lobus caudalis cerebelli. (d) Experimental paradigm for delayed classical fear conditioning. In the habituation session, CS alone was given for 5?s per trial; 10?15 trials were performed. In the acquisition session, a paired CS and US (1-ms electric shock given 4?s after the CS onset) was given in each trial; 20 trials were performed. In the probe session, the CS alone was given in each trial and 10 trials were performed. As a control (backward conditioning), the US was given 2?s before the CS onset in the acquisition session. Trials were separated by a 50-s interval. A 50-s interval was also provided between sessions. |
|
Granule-cell inhibition prolonged conditioned fear responses. (a) Schematic of granule-cell inhibition. Tg larvae with inhibited granule-cell activity (GC-silenced larvae) were obtained by crossing the granule-cell-specific Gal4 line gSA2AzGFF152B and the Tg(UAS:BoTxBLC-GFP) line, which expresses a fusion protein of GFP and the light chain of botulinum toxin light B (BoTxBLC-GFP) in a Gal4-dependent manner. (b?h) BoTxBLC-GFP expression in 20-dpf GC-silenced larvae. Immunostaining of the brain (whole-mount, b?d) and sagittal sections (e?h) with anti-GFP (green) and Neurod1 (magenta) antibodies, showing the cerebellar regions. Dorsal views with anterior to the left. (f?h) Higher magnification views of the dotted box in (e). Neurod1 signals mark granule-cell nuclei. About a half of the mature granule cells in the CCe (located in the granule-cell layer) expressed BoTxBLC-GFP in the GC-silenced larvae (Supplementary Fig. S3, Table S1). Scale bars: 100 ?m in (b) (applied to c,d); 100 ?m in (e); 20 ?m in (f) (applied to g,h). GCL, granule-cell layer; ML, molecular layer (see Fig. 1 for other abbreviations). (i) Percentage of fish showing CS-dependent bradycardia (learner fish); 15 of the 40 wild-type (WT) larvae were learners, 16 of the 50 GC-silenced larvae were learners, and 20 of the 58 sibling GFP-negative larvae were learners. There was no significant difference in the learner rate among wild-type, the GC-silenced and the sibling groups (P?=?0.8615, Fisher?s exact test). ns represents no significance. (j) CS-evoked bradycardia responses in wild-type, the GC-silenced, and their sibling learner fish. Relative HB frequency during 1 s period of the 5?s CS presentation (average of each one-second block) is shown. Average of the data from 10 trials in the habituation and probe sessions was calculated and plotted in graphs. The graphs show the average and standard errors (SE) of the data from wild-type larvae (n?=?15, gray dotted), the GC-silenced larvae (n?=?16, red), and their sibling larvae (n?=?20, black). The CS-evoked bradycardia responses during the probe session differed between the GC-silenced and the sibling learner groups (** represents P?<?0.01 and * represents P?<?0.05, two-way repeated measures ANOVA with Bonferroni?s post-hoc test). Both the GC-silenced and sibling larvae exhibited bradycardia in response to the CS during the probe session. The bradycardic response of the GC-silenced larvae was prolonged in the probe sessions. Note that the change of relative HB frequency in wild-type learner fish during the habituation and probe sessions was similar to that in the sibling learner fish. |
|
Cerebellar neurons were activated during classical fear conditioning. (a,b) Ca2+ imaging. GCaMP7a fluorescence intensity in Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) larva before (pre-CS, a) and during (dur-CS, b) the CS in the probe sessions. The images were taken 2?s before and 2.5?s after the start of the CS presentation. Average images from nine trials in the probe session are shown. (c) The ?dur-CS minus pre-CS? image generated by subtraction. Note that the CS evoked upregulated fluorescence intensity only in the CCe. (d,e) Time course of cerebellar neuronal activity. The fluorescence intensity from nine cerebellar neurons in a single larva was monitored during the habituation (11th?15th trials) and probe (1st?5th trials) sessions. Graph shows the ?F/F, which was calculated by dividing the change in fluorescence intensity at the indicated time point by the average intensity at 2?s before the CS (e). Gray boxes indicate the timing of the CS presentation. The position of the neurons is shown in (d). During the probe session, the CS evoked upregulated fluorescence in seven neurons (Cells 1?7) in the CCe. There was no change in fluorescence between the habituation and probe sessions in a neuron in the EG (Cell 8) or in one in the LCa (Cell 9). (See Fig. 1 for abbreviations). |
|
Two types of conditioning-associated neurons. We found two types of conditioning-associated neurons that were activated by the CS. Type I neurons responded quickly to the CS, while type II neurons were activated after a delay. (a) Neuronal activity (?F/F) of type I and II neurons of Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) larvae during the probe session (1st?5th trials), showing data from five type I neurons and two type II neurons. Gray boxes indicate the timing of the CS presentation. (b) Location of the type I (red dots) and II neurons (blue dots) in the CCe. These neurons were located close to each other in the CCe. (c?f) Immunostaining of Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) about 20-dpf larval brains with anti-GFP (green) and anti-Neurod1 (magenta) antibodies. (d?f) Higher magnification views of the dotted box in (c). The inset in (f) is a higher magnification view of the dotted box in (f). Neurod1 signals mark granule-cell nuclei. Note that Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) fish expressed GCaMP7a in most of the granule cells in the Va/CCe/LCa (Va: valvula cerebelli) (white asterisks in the inset in f). (g?j) Immunostaining of Tg(elavl3:GAL4-VP16); Tg(UAS:GCaMP7a) brains with anti-GFP (green) and anti-Pvalb7 (magenta) antibodies. (h?j) Higher magnification views of the dotted box in (g). The inset in (j) is a higher magnification view of the dotted box in (j). Pvalb7 signals mark both the neurites (axons and dendrites) and somata of Purkinje cells. No Purkinje cells expressed GCaMP7a (Table S2). Pvalb7+ cells in the GCL are also Purkinje cells as migration of Purkinje cells was not completed at the stage. See Figs 1 and 3 for abbreviations. Scale bars: 50 ?m in (c) (applied to g); 20 ?m in (d) (applied to d?f, h?j); 10 ?m in the inset in (f) (applied to the inset in j). |