- Title
-
The zebrafish tailbud contains two independent populations of midline progenitor cells that maintain long-term germ layer plasticity and differentiate in response to local signaling cues
- Authors
- Row, R.H., Tsotras, S.R., Goto, H., Martin, B.L.
- Source
- Full text @ Development
|
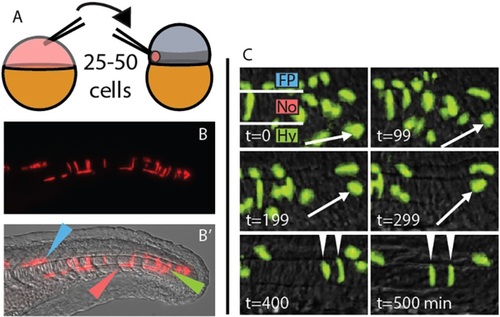
A midline-directed transplant technique reveals long-lived MPCs. (A) A heterochronic cell transplantation scheme, from transgenic or transiently transgenic donors to wild-type zebrafish hosts, maximizes contribution to the MPCs. 25-50 cells are taken from a dye-injected sphere stage donor and placed in the prospective mesendoderm (darker gray band) of a 1000-cell stage host. (B,B′) At 24h post fertilization (hpf), transplanted cells are observed exclusively in the derivatives of the MPCs. Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively. (C) Transplanted cells can remain undifferentiated for an extended time, and can join the notochord, hypochord and floor plate of the neural tube. Arrow marks one cell that divides and differentiates into two notochord cells (arrowheads). t=0 is ~18 hpf. Fp, floor plate; No, notochord; Hy, hypochord. |
|
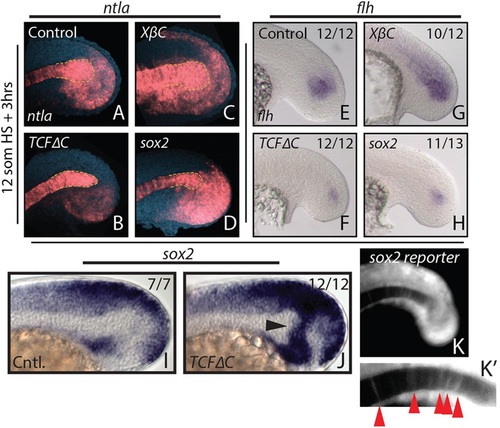
Canonical Wnt signaling affects tailbud notochord progenitor fate through sox2 repression. (A-H) Heat shock-inducible transgenic lines were used to manipulate canonical Wnt signaling or sox2 expression after gastrulation at the 12-somite stage, and stained for ntla or flh expression 3 h after the heat shock. Loss of Wnt signaling causes a reduction in ntla expression specifically in the notochord progenitor domain (A,B, yellow dashed line indicates the progenitor domain), as well as a reduction in the notochord progenitor marker flh (E,F). Activation of Wnt signaling has the opposite effect on notochord progenitors (C,G). (I,J) sox2 is normally expressed in regions directly adjacent to the notochord progenitor domain (I) and expands dramatically into the notochord progenitor domain 2h after loss of Wnt signaling at the 12-somite stage (J, arrowhead). Heat shock induction of sox2 expression phenocopies Wnt loss of function with respect to ntla (D, dashed yellow line) and flh (H) expression. A sox2 reporter line shows weak fluorescence in notochord cells at the 16-somite stage (K,K′, arrowheads), indicating that notochord cells were once sox2 positive. The number of embryos showing the illustrated phenotype among the total number examined is indicated. |
|
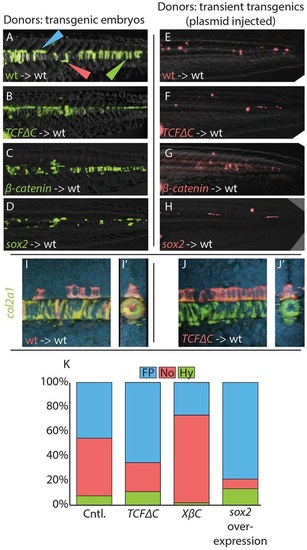
Cell fate distributions are affected by changes in Wnt signaling or sox2 overexpression. (A-H) Cells from stable transgenic donors (A-D) or from transiently transgenic donors (E-H) were transplanted into wild-type hosts and transgene expression induced after the completion of gastrulation (bud stage). (I-J′) In some cases, host embryos were stained by fluorescent in situ hybridization for col2a1 expression (green) and imaged by confocal microscopy. Transplanted cells are in red. A maximum projection image (I,J) and digital transverse section (I′,J′) reveal the precise midline position of transplanted cells from control (I,I′) and hsp70l:TCFΔC-GFP (J,J′) transplanted cells. (K) The contribution of transient transgenic cells to floor plate, notochord and hypochord was quantitated (raw data and statistics are provided in Table 1). Blocking Wnt signaling (B,F,J,J′) expanded the floor plate contribution at the expense of notochord, and activating Wnt had the opposite effect (C,G). Overexpression of sox2 (D,H) produced an effect very similar to blocking Wnt. Cell fate changes are statistically significant (see Table 1). Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively. |
|
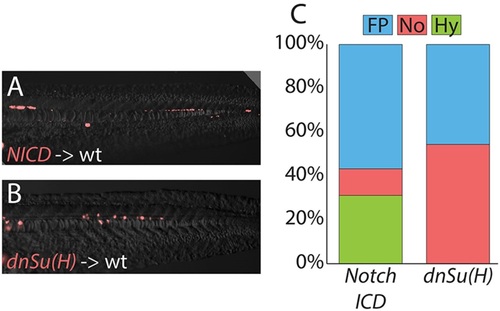
MPCs remain responsive to changes in Notch signals after gastrulation. Transiently transgenic cells were transplanted into wild-type host embryos, and transgene expression activated after gastrulation had completed. Activated Notch signaling cell-autonomously promotes hypochord and floor plate fates at the expense of notochord (A), and Notch activity is required for cells to adopt a hypochord fate (B), with changes quantitated (C; raw data and statistics are provided in Table 1). Cell fate changes are statistically significant (see Table 1). |
|
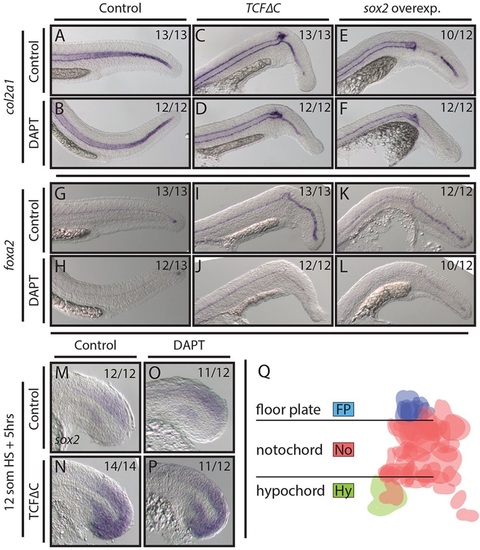
Wnt and Sox2 pattern midline tissues independently of Notch activity. (A-L) Loss of Wnt signaling and overexpression of sox2 using stable transgenic lines causes the notochord domain of col2a1 expression to be lost in the tail (A,C,E), whereas the floor plate marker foxa2 shows enhanced expression (G,I,K). Combining DAPT treatment with these conditions immediately after the heat shock has no effect on notochord patterning (B,D,F) but does reduce foxa2 expression (H,J,L). (M-P) Blocking Wnt signaling results in ectopic sox2 expression throughout the tailbud (M,N). This effect is independent of active Notch signaling (O,P). (Q) A fate map of MPCs (see text for details) indicates that floor plate cells are exclusively derived from a dorsal MPC population (blue), whereas hypochord (green) is derived only from a ventral MPC population. Cells are false colored based on fate; horizontal lines indicate the boundary of the notochord. |
|
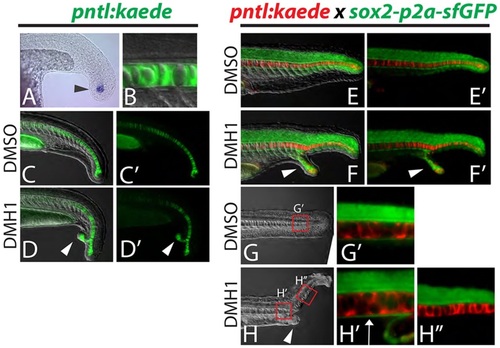
Dorsal and ventral MPCs separate in embryos with ectopic tails. (A-D′) A newly created transgenic reporter line (pntl:kaede) exhibits kaede mRNA expression in notochord progenitor cells (A, arrowhead) and Kaede protein perdures in the notochord only (B). Ectopic tails always contain notochord cells (D,D′, arrowheads). (E-H′′) Notochord and hypochord were visualized simultaneously by crossing the pntl:kaede line to the sox2-p2a-sfGFP line and photoconverting the Kaede from green to red. Analysis of ectopic tails at 24hpf revealed that the hypochord is diverted from the primary midline into the ectopic tail (F,F′, arrowheads). At 36hpf it is clear that the hypochord is present anterior to the ectopic tail (ectopic tail is pictured in H, arrowhead) as labeled by sfGFP driven by the sox2 locus (H2, arrow), but is absent in the primary midline posterior to the ectopic tail (H′′). |
|
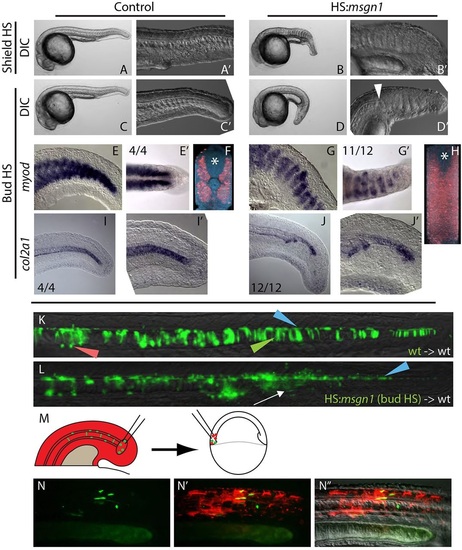
Local signals regulate MPC versus PWPC fate. (A-B′) Heat shock induction of msgn1 at the start of gastrulation (shield stage) results in the absence of notochord and the ectopic expansion of somites across the midline at 24hpf. (C-D′) Heat shock induction of msgn1 after gastrulation (bud stage) causes the notochord to end abruptly, followed posteriorly by the expansion of somites at the midline at 24hpf. The arrowhead indicates the transition from notochord to somite at the midline. (E-J′) In embryos heat shocked at bud stage, myoD expression at 24hpf is present in posterior regions of the embryo (E,G) and, when viewed dorsally, can be seen extending across the midline (E′,G′). Fluorescent in situ hybridization of myoD expression reveals (by digital transverse section) the absence of notochord and presence of muscle across the midline (F,H, asterisk marks the position of the spinal cord). Expression of col2a1 indicates that the notochord is truncated (I-J′). (K,L) Ectopic msgn1 expression at bud stage cell-autonomously converts some MPCs to a somitic fate, as seen in transplanted cells. Control donor cells only contribute to floor plate, notochord and hypochord (K); somite contribution was observed in 1/16 embryos. By contrast, HS:msgn1 donor cells transition from notochord contribution to somite contribution (L, white arrow; notochord to somite switch was observed in 12/16 embryos) without affecting floor plate contribution. Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively. (M-N′′) A serial transplantation scheme (M) was used to target MPCs to the PWPCs. MPCs (green) from the 12-somite stage tailbud are able to contribute to skeletal muscle tissue when analyzed at 24hpf (N-N′′). |
|
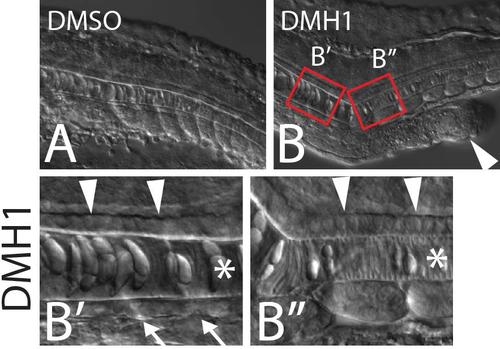
Embryos treated with the BMP inhibitor DMH1 exhibit ectopic tails (B, arrowhead). High magnification views of the primary midline reveal that in regions anterior to the ectopic tail, floor plate (arrowheads), notochord (star), and hypochord (arrows) are all present (B′), whereas posterior to the ectopic tail hypochord is absent from the primary midline but floor plate is present (B′′). |