- Title
-
Regulation of Sufu activity by p66β and Mycbp provides new insight into vertebrate Hedgehog signaling
- Authors
- Lin, C., Yao, E., Wang, K., Nozawa, Y., Shimizu, H., Johnson, J.R., Chen, J.N., Krogan, N.J., Chuang, P.T.
- Source
- Full text @ Genes & Dev.
|
Complex formation between Sufu, p66β, Mycbp, and Gli proteins and their dynamic interactions on the promoters of Hh-responsive genes. (A) Western blot analysis of immunoprecipitates using lysates from MEFs expressing Flag-tagged Gli2 or Gli3. Endogenous p66β and Sufu were coimmunoprecipitated, consistent with the formation of a Sufu/p66β/Gli protein complex. (B) Western blot analysis of immunoprecipitates using lysates from Sufu-deficient MEFs expressing Flag-tagged Gli2 or Gli3. Endogenous p66β failed to be coimmunoprecipitated, suggesting that Sufu bridges the interaction between p66β and Gli protein. (C) Western blot analysis of immunoprecipitates using lysates from MEFs expressing Flag-tagged Mycbp. Endogenous Sufu, Gli2, and Gli3 were coimmunoprecipitated, implying the production of a Sufu/Mycbp/Gli protein complex. Moreover, Mycbp dissociated from Sufu upon Hh treatment, while Mycbp interaction with full-length Gli2 and Gli3 was enhanced upon Hh pathway activation. (*) P < 0.05; (**) P < 0.01; (NS) not significant (unpaired Student’s t-test) (n = 3). Mycbp/Gli3 interaction was increased upon Hh stimulation, but variations in immunoprecipitations affected the calculated statistical value. (D) Immunoprecipitation of Gli2, Gli3, and Sufu using oligonucleotides that contain a canonical GliBS or control oligonucleotides in which the GliBS is mutated (denoted as ΔGliBS). Sufu, full-length (FL) Gli2, full-length Gli3, and Gli3R were immunoprecipitated by a GliBS without Hh stimulation but not by the control ΔGliBS. Hh stimulation led to an increased binding of Gli2 and Gli3 to the GliBS, while association of Sufu or Gli3R with the GliBS was weakened. (E) Sufu failed to be immunoprecipitated by the GliBS in Gli2-/-; Gli3-/- MEFs, indicating that Sufu binding to the GliBS is dependent on Gli proteins. (F) ChIP analysis of Gli1, Gli2, Sufu, p66β, and Mycbp on the promoter of Hh-responsive genes such as Ptch1. Gli1, Gli2, Sufu, and p66β proteins were enriched on the Ptch1 promoter by ChIP. Hh treatment led to the reduced presence of Sufu and p66β on the Ptch1 promoter, while binding of Mycbp to the Ptch1 promoter was enhanced. Interestingly, enhanced binding of Mycbp to Hh target gene promoters was abolished in Gli2-/-; Gli3-/- MEFs (Supplemental Fig. S24), suggesting that the promoter occupancy of Mycbp is dependent on Gli proteins. (*) P < 0.05 (unpaired Student’s t-test) (n = 3). |
|
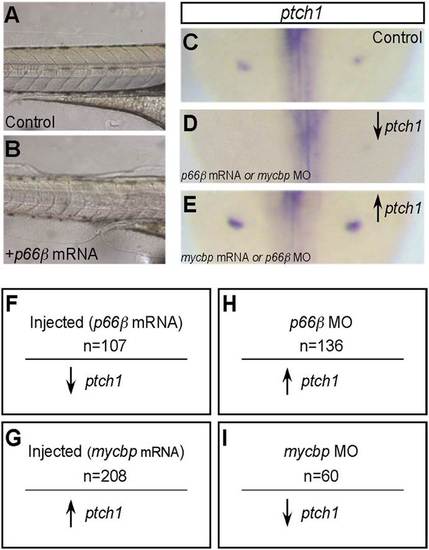
Expression of p66β and mycbp in zebrafish disrupts Hedgehog (Hh) signaling and results in somite defects. (A) Lateral view of chevron-shaped somites in wild-type zebrafish embryos at 24 hours postfertilization (hpf). (B) Zebrafish embryos injected with p66β exhibited U-shaped somites, consistent with disruption of Hh signaling. (C) Zebrafish embryos injected with mycbp also displayed U-shaped somites, known to be associated with disruption of Hh signaling. Reduced or increased Hh signaling can both produce U-shaped somites. |