- Title
-
The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase
- Authors
- Guenebeaud, C., Goldschneider, D., Castets, M., Guix, C., Chazot, G., Delloye-Bourgeois, C., Eisenberg-Lerner, A., Shohat, G., Zhang, M., Laudet, V., Kimchi, A., Bernet, A., and Mehlen, P.
- Source
- Full text @ Mol. Cell
|
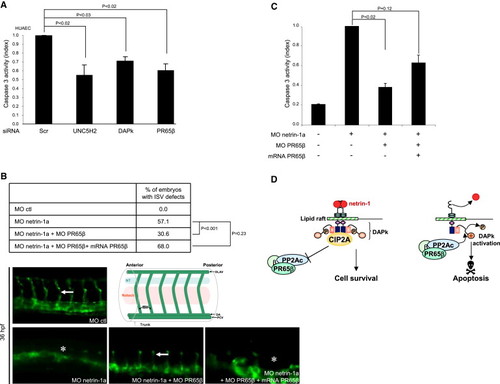
PP2A Is Involved in UNC5H2 Proapoptotic Signaling during Angiogenesis (A) UNC5H2, DAPk, and PR65β siRNA silencing by siRNA are associated with a decrease in caspase-3 activity in HUAEC endothelial cells. Results are means ± SEM. (n = 3). All p values are < to 0.03 (U test). (B) Silencing of PR65β using specific morpholinos rescues vascular defects induced by netrin-1a silencing in fli:eGFP zebrafish embryos. PR65β morpholinos rescue the effect of netrin-1 withdrawal-induced defects, abolished by injection of PR65β mRNAs. Intersegmental vessel (ISV) presence was analyzed at 30 hr gestation. Representative images of morpholino-injected zebrafish embryos are shown for each condition. Percentage of zebrafish with ISV defect is indicated (p < 0.001, Khi-2 test). (C) PR65β silencing reduces caspase-3 activation induced by netrin-1a silencing in zebrafish embryos. Injection of PR65β mRNAs suppress this effect. Results are means ± SEM of caspase-3 activity (n = 3; p < 0.02, Student′s t test). (D) Model of PR65β/PP2A and CIP2A implication in DAPk-mediated UNC5H2-induced apoptosis. In the presence of netrin-1, the UNC5H2 receptor adopts a closed conformation and interacts with an inactive, phosphorylated form of DAPk. Phosphorylation of DAPk is maintained by CIP2A, which interacts with UNC5H2 and inhibits PP2A. In the absence of netrin-1, UNC5H2 adopts an open conformation and recruits PR65β/PP2A into an UNC5H2-DAPk complex. PR65β/PP2A recruitment leads to DAPk dephosphorylation and activation, and thus to apoptosis induction. |