- Title
-
Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development
- Authors
- Galindo-Villegas, J., García-Moreno, D., de Oliveira, S., Meseguer, J., and Mulero, V.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
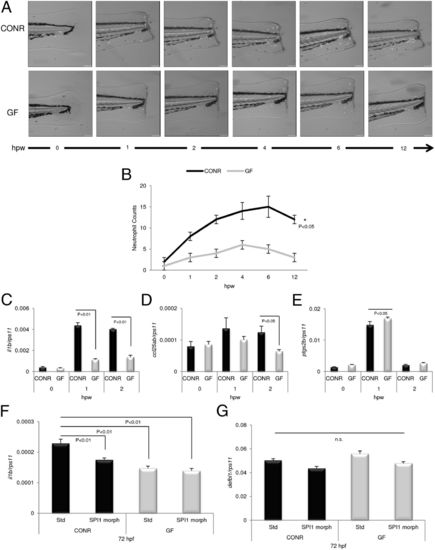
Microbial presence increases neutrophil recruitment in vivo and induces the expression of proinflammatory mediators. Tail fins of Tg(mpx::eGFPi114) CONR and GF zebrafish were transected at 72 hpf, and the number of fluorescent neutrophils visible in the tail was assessed by fluorescence microscopy. (A) Representative images of positive neutrophils expressing eGFP recruited to wounding site in a CONR and a GF zebrafish larva. hpw, hours postwounding. (B) Positive cells in animals from both groups were quantified visually from high-quality pictures from a 12-h time series. Inflammatory events were significantly faster, stronger, and took more time to resolve in the CONR group than in GF embryos (*P < 0.05). n = 50 larvae per group and sampling point. (C?E) At the time points indicated, 80 individual fish were anesthetized and then a sterile scalpel was used to incise the body between the anus and the wounded tail tip. Individual samples of the same batch were pooled and were immersed immediately in TRIzol for quantification of IL-1β (C), CCL-C25ab (D), and PTGS2B (E) mRNA levels by qPCR. (F and G) Zebrafish eggs were microinjected at the one-cell stage with 8 ng STD or SPI morpholinos per egg and then were divided into two batches. One batch was raised as CONR, and the other was derived as GF. IL-1β (F) and DEFBL1 (G) transcript levels, assayed by qPCR, of 72-hpf SPI morphants and their STD morphant siblings, raised CONR or GF. Error bars indicate the SD of three independent experiments, each using 30 pooled larvae per treatment. n.s., nonsignificant. EXPRESSION / LABELING:
|
|
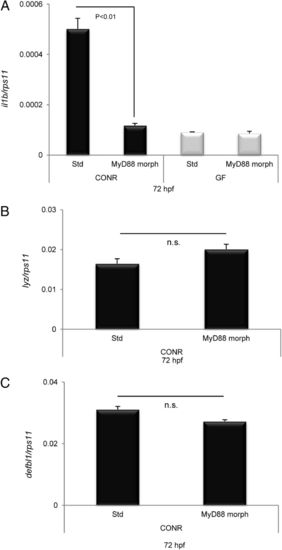
MAMPs signal through a TLR/MyD88 signaling pathway. Zebrafish eggs were microinjected at the one-cell stage with STD or MyD88 morpholinos, 4 ng per egg, and then were divided into two batches. One batch was raised as CONR, and the other was derived as GF. IL-1² (A), LYZ (B), and DEFBL1 (C) transcript levels, assayed by qPCR, of 72-hpf MyD88 morphant zebrafish and their STD morphant siblings raised CONR or GF. Error bars indicate the SD of two independent experiments, each using 30 pooled larvae per treatment. n.s., nonsignificant. EXPRESSION / LABELING:
|
|
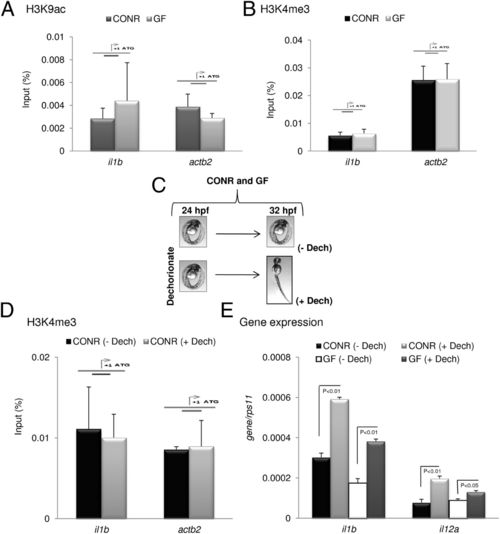
Histone modifications are not regulated by commensals or hatching. (A and B) Naturally hatched CONR and GF zebrafish larvae were analyzed by ChIP (H3K9ac and H3K4me3) at 72 hpf. (C) Scheme showing the generation of CONR and GF embryos after manual dechorionation. These embryos were analyzed by ChIP (H3K4me3) (D) or gene expression (IL-1β and IL-12a) (E) at 32 hpf. The amplicon used for each promoter is indicated by a diagram above the bars. Error bars indicate the SD of two independent experiments, each using 30 pooled larvae per treatment. |