- Title
-
Function of FGF signaling in the developmental process of the median fin fold in zebrafish
- Authors
- Abe, G., Ide, H., and Tamura, K.
- Source
- Full text @ Dev. Biol.
|
(A, B) Overview of the median fin fold in a 42 hpf zebrafish embryo from the lateral view (A) and higher magnification of anterior trunk region (B). The continuous midline fin fold (mff, median fin fold (MFF)) surrounds the tail and trunk region from the cloaca (arrow in panel A) to the rostral edge at the 8th somite level (arrow in panel B). paff, pre-anal fin fold; y, yolk; ye, yolk extension. Numbers indicate the somite number. (C?H, a?l) Histological analysis of median fin fold. (C?H) 15?24 hpf zebrafish embryos from the lateral view. (a?l) HE staining of transverse sections at the level of each bar in panels C?H. At 15 hpf (C, a) and 16 hpf (D, b), the presumptive fin fold epidermis (indicated by the black broken lines in a and b that are under the epidermal cell layer) was still connected to the neural ectoderm in the tail bud. (E, c) Epidermis covered the midline of the tail and trunk at 17 hpf (indicated by the black broken lines in c). ep, epidermis; nc, notochord; nt, neural tube; pe, peridermis; pnt, prospective neural tube; so, somite. (F) At 18 hpf, wedge-shaped epidermal cells were distinguishable at the midline of the tail bud (e, f; indicated by brackets), but these cells were not observed at a more rostral region (d). (G, H) Then the area in which wedge-shaped cells were seen (h, i for 21 hpf, and k, l for 25 hpf; indicated by brackets) expanded rostrally and caudally as the tail elongated. The dots indicate the final position of the anterior boundary of the fin fold (8th somite level). (j2, f2) High magnifications of j and f. Typical cuboidal-shaped epidermal cells and wedge-shaped epidermal cells indicated by the blue (in j2) and red (in f2) broken lines, respectively. Scale bars in panels A?C, F, and (a) are 500 μm, 100 μm, 500 μm, 200 μm, and 50 μm, respectively. The panel width of (d?l) is 100 μm. |
|
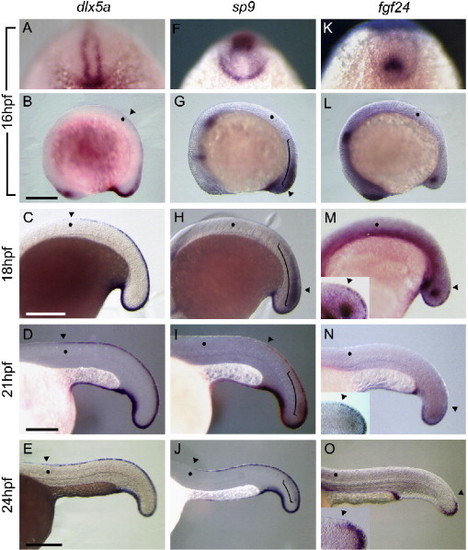
Expression patterns of some marker genes during development of the median fin fold. Expression patterns of dlx5a (A?E), sp9 (F?J), and fgf24 (K?O): lateral view (B?E, G?J, and L?O) with head toward the left, and tail view (A, F, and K) with dorsal midline toward the top. Insets in panels M?O show higher magnifications focused on the dorsal half of the tail bud corresponding with the region in panels M?O, respectively; lateral view with rostral to the left. Arrowheads indicate the rostral border of the expression domain. Dots indicate the 8th somite level. (A) dlx5a was expressed around the tail bud and in the epidermal cells juxtaposed with the neural keel at 16 hpf. (B) At this stage, dlx5a expression was seen on the dorsal midline at the 8th somite level and caudally. (C?E) The rostral border of the dlx5a expression domain remained around the 8th somite level at 18 hpf (C), 21 hpf (D), and 24 hpf (E). (F, G) At 16 hpf, sp9 expression was observed in neural tissue (indicated by the bracket) and non-neural epidermis around the tail bud. At this stage, sp9 expression was restricted to the tail bud, and the more anterior dorsal midline was sp9-negative. (H?J) The rostral border of the sp9 expression domain expanded toward the head as development proceeded and the tail bud elongated. Compare the position of the arrowhead with the position of the 8th somite indicated by a dot. (K, L) fgf24 expression was seen in the mesenchyme of the tail bud and intermediate mesoderm at 16 hpf. (M?O and insets) fgf24 expression in the epidermal cells was always restricted to the midline of the tail bud, and there was no expansion of the expression domain of fgf24. Scale bars are 250 μm in panels B, C, and E and 125 μm in panel D. EXPRESSION / LABELING:
|
|
SU5402 inhibits extension of the MFF structure formation. Embryos were exposed to 0.17% DMSO for control (A, C, E, G) or 20 μM SU5402 (B, D, F, H) from 18 hpf for 3 h. (A, B) Lateral view of embryos, anterior to the left. (a, b, c, d) HE staining of transverse sections at the level of each bar in panels A and B. While the dorsal midline (at the level of yolk extension) of control embryos consisted of wedge-shaped cells (a; bracket), SU5402-treated embryos did not have such cells at the same level (c). At the tail bud, the fin fold was seen in SU5402-treated embryos (d; bracket) but was immature compared with that in control embryos (b; bracket). (C?H) Expression patterns of dlx5a (C, D), sp9 (E, F), and fgf24 (G, H). (D) dlx5a expression was unaffected in SU5402-treated embryos. (F) Expression of sp9 was suppressed in the median fin fold after SU5402 treatment, and the expression domain was limited caudally to the cloaca level (arrowhead). Expression of fgf24 was not detectable in the tail tip of SU5402-treated embryos (H). Arrowheads in panels C?F indicate rostral border of the expression domain. Scale bars in panels A and C are 200 μm. The panel width of (a?d) is 100 μm. EXPRESSION / LABELING:
|
|
SU5402 did not affect dlx5a expression in the presumptive fin fold epidermis. Embryos were exposed to 0.17% DMSO for control (A, C, E, G, I) or 20 μM SU5402 (B, D, F, H, J) from 15 hpf for 3 h. Lateral view of embryos, anterior to the left. (a, b) HE staining of transverse sections at the level of each bar in panels A and B. Bracket in (a) indicates the area in which wedge-shaped cells were seen. The dorsal midline in the SU5402-treated embryo (b) was not covered with epidermis (arrowheads), and wedge-shaped cells for the fin fold were not formed. (C?F) In SU5402-treated embryos, dlx5a expression was retained as bilateral stripes in epidermal cells juxtaposed with the neural keel (D, F). (G?J) Expression patterns of sp9 (G, I) and fgf24 (H, J). Neither sp9 (H) nor fgf24 (J) was detected in the dorsal midline of SU5402-treated embryos. Bracket in (G, I) indicates sp9 expression observed in neural tissue. Scale bars in panels A and C are 200 μm. The panel width of (a, b) is 100 μm. EXPRESSION / LABELING:
|
|
Ectopic median fin fold-like structure formation was induced by FGF application to the dorsal midline. (A?E) Lateral view of the anterior trunk region in 42 hpf or 24 h post implantation embryos. FGF-soaked beads were implanted into the dorsal midline at around the second and third somite level of 18 hpf embryos. Arrows indicate the anterior edge of the median fin fold and ectopic median fin fold-like structure. (A) Control embryos usually had the anterior end of the median fin fold at the 8th somite level. (B?E) Ectopic median fin fold-like structure could be seen rostrally to the intrinsic median fin fold after implantation of FGF beads (B; FGF4, C; FGF7, D; FGF8, E; FGF10). Arrowheads in panel B indicate ectopic hypertrophy that was disconnected from the intrinsic fin fold. (F) The ratio of the position of the anterior end of the median fin fold-like structures in the bead-implanted embryos. The colors of bars in the graph indicate the position of the anterior end in the median fin fold and the median fin fold-like structure: yellow is somite 7?8 level, orange is somite 5?6 level, and maroon is somite 4< region. The colors of bars correspond to the colors of arrows in panels A?E. |
|
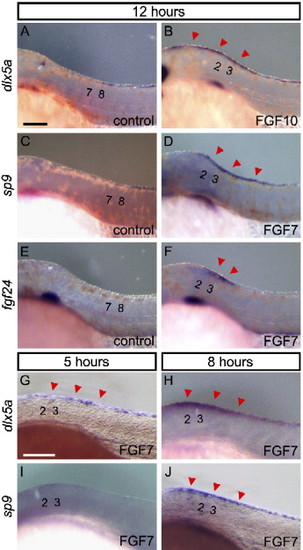
Expression of marker genes for the median fin fold in ectopic structure formation induced by FGF7/10. Expression of genes in control bead-implanted embryos (A, C, E) and FGF7/10 bead-implanted embryos (B, D, F) at 12 h after implantation of beads. (A, B) dlx5a, (C, D) sp9, dlx5a (B), and sp9 (D) are expressed in the ectopic structure, continuously from the normal domain. (E, F) fgf24, which is normally expressed only in the tail bud, is (F) also seen ectopically but restricted to the epidermis around the bead. (G?J) Onset of the expression of dlx5a and sp9; dlx5a expression was detected from 5 h after implantation of beads (G, H), while sp9 expression was not detected at 5 h after (I) but was detected at 8 h after implantation of beads (J). Red arrowheads indicate an additional domain of gene expression. Numbers indicate the somite number. Scale bars in panels A and G are 100 μm. |
Reprinted from Developmental Biology, 304(1), Abe, G., Ide, H., and Tamura, K., Function of FGF signaling in the developmental process of the median fin fold in zebrafish, 355-366, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.