- Title
-
A two-step mechanism underlies the planar polarization of regenerating sensory hair cells
- Authors
- Lopez-Schier, H., and Hudspeth, A.J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
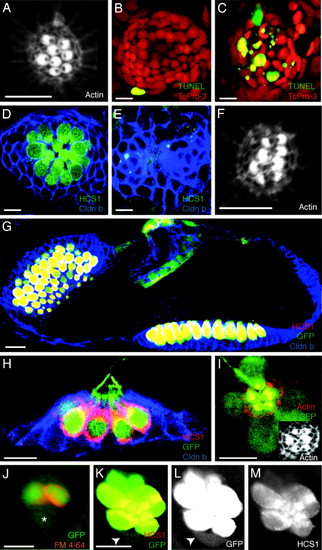
Characterization of hair-cell death and ET4 transgenic zebrafish. (A) In a confocal image of a neuromast in a normal 3-day-old larva, stereocilia are labeled with rhodamine-phalloidin to reveal the polarization of hair bundles toward the animal′s head or tail. (B) In a control larva, only one peripheral cell displays TUNEL staining (green) indicative of apoptosis. To-Pro-3 labeling (red) marks all nuclei. (C) Neomycin treatment increases the number of apoptotic cells at the center of the neuromast. (D) In an untreated animal, immunolabeling with the hair cell-specific marker HCS1 (green) identifies hair cells, and claudin b labeling (blue) designates supporting cells. (E) A neomycin-treated larva lacks hair cells. (F) In a regenerating L1 neuromast, actin staining shows that hair bundles orient along the neuromast′s original, anteroposterior axis of planar cell polarity. (G) GFP-positive hair cells (green) in the ear of a 3-day-old ET4 transgenic animal are also positive for the specific hair-cell marker HCS1 (red). Claudin b immunoreactivity (blue) marks supporting cells. (H) In a lateral view of a neuromast from an ET4 transgenic animal labeled for claudin b (blue) and HCS1 (red), GFP-positive hair cells (green) are also marked by HCS1. (I) Labeling stereocilia with rhodamine-phalloidin (red) confirms that planar cell polarity is normal in an ET4 larva, whose hair cells express GFP (green). (I Inset) Only the actin staining is shown. (J) Incubation of an ET4 larva in FM4?64 (red) reveals a single, weakly GFP-positive cell (asterisk) that does not incorporate this vital dye. (K?M) Labeling of an ET4 larva with HCS1 (red) shows a gradient of intensity for this specific hair-cell marker and for GFP (green) toward the periphery of the neuromast. A single weakly GFP-positive cell at the lower edge of the neuromast (arrowheads) is negative for HCS1. In these and all subsequent figures, the animal′s anterior is oriented to the left and its dorsum is situated to the top. (Scale bars: 10 μm.) EXPRESSION / LABELING:
|
|
Time-course analysis of hair-cell production. (A?H) In a 12-h-long series of confocal images begun 2 days after fertilization, labeling of an ET4 larva with Texas red-ceramide (red) reveals two GFP-positive mature hair cells (yellow). At the lower edge of the neuromast, a pair of immature hair cells (green; arrows in C), whose proximity initially makes them appear confluent, separate over the course of 3 h (B and C) and become yellow as they mature (D?H). A hair-cell precursor meanwhile develops at the upper edge of the neuromast (arrowhead in B). Over the next hour, this precursor increases its green fluorescence and commences mitosis; the absence of GFP identifies the chromosomes congregated in the metaphase plate (D) and segregated in anaphase (E). The daughter cells (arrowheads in F) eventually separate to form two hair cells (H). This time-lapse series and the supporting movies indicate that hair cells develop in pairs along a single axis in neuromasts. (I) Only 6 h after ablation of hair cells in a wild-type larva, actin staining delineates the first, immature pair of hair bundles. (J) By 10 h after treatment, a neuromast has developed three mature pairs of hair bundles whose opposing polarities are apparent. (K) A neuromast at 16 h after treatment shows the orientation of four pairs of hair bundles. (L) In another animal at the same stage of recovery, five pairs of hair bundles demarcate a line of symmetry dividing the neuromast into two halves along the dorsoventral midline. All of the hair bundles on each side of the line of symmetry have the same orientation. (M) A composite of two confocal images of a regenerating ET4 animal, in which the apical surfaces of the hair cells are pseudocolored red and the cell bodies green, shows that the line of symmetry defined by the hair bundles corresponds to the arrangement of cell bodies. Two immature hair cells have not yet developed hair bundles (arrowheads). (N) By 40 h after treatment, the original line of symmetry is still evident (dotted yellow line), but a second line also has appeared on one side of the neuromast (dotted green line). Three unpaired hair cells are marked with red asterisks. (Scale bars: 10 μm.) |
|
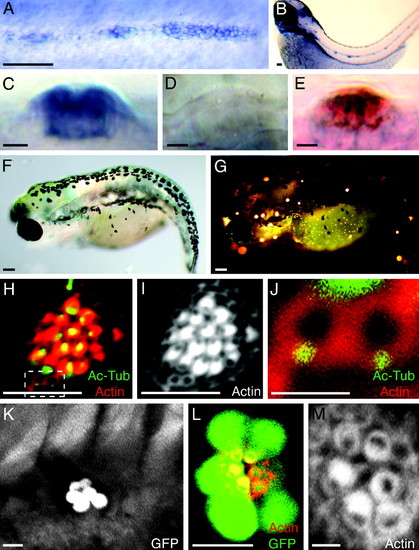
Random orientation of hair bundles in neuromasts of trilobite mutant zebrafish. (A) In situ hybridization shows that vangl2 is expressed by the migrating primordium of the posterior lateral line in a 2-day-old wild-type embryo. (B) Expression is apparent in mature neuromasts at 3 days of age. (C) In a lateral view of a mature neuromast, the labeling indicates that the vangl2 transcript is expressed strongly by most neuromast cells. (D) A trilobitevu7 deletion mutant lacks vangl2 labeling. (E) A two-color in situ hybridization for vangl2 (blue) and the hair-cell marker parvalbumin 3a (red) indicates that vangl2 is expressed by both hair cells and supporting cells. (F) A brightfield micrograph displays the shortened, curled body of a trilobitem209 mutant larva at 5 days of age. (G) Fluorescence microscopy after exposure to 4-Di-2-Asp reveals that the trilobite larva possesses functional neuromasts along both the anterior and the posterior branches of its lateral-line system. (H?J) A neuromast of a 5-day-old trilobitem209 larva labeled for acetylated α-tubulin (green) and actin (red) shows that hair bundles assume random orientations. Two young hair cells occur at the lower edge of the neuromast (dashed box). (I) Staining of actin alone accentuates the defect in planar cell polarity. (J) A high-magnification view of the same neuromast reveals the mislocalization of basal bodies in two, probably sibling hair cells that have not yet formed mature hair bundles. (K) A low-magnification view of the trunk of an ET4;trilobite larva demonstrates that hair-cell pairs are aligned along a single axis in a neuromast. (L) A high-magnification view of a regenerating neuromast in an ET4;trilobite animal expressing GFP (green) and stained for actin (red) shows the alignment of hair cells along the neuromast′s line of symmetry. (M) In a higher-magnification view of this neuromast, actin staining demonstrates that, although the hair bundles respect the line of symmetry, they are misoriented with respect to one another. (Scale bars: A, B, F, and G, 100 μm; C?E, H, I, K, and L, 10 μm; J and M, 2 μm.) |
|
Characterization of hair-cell regeneration in several zebrafish mutant lines. (A?C) Gastrulation defects in zebrafish are associated with the silverblick/wnt11, pipetail/wnt5a, and knypek mutations (21?23). Labeling of neuromasts in 6-day-old larvae with rhodamine-phalloidin reveals that the hair bundles are properly orientated in pipetail (A), silberblick (B), and knypek mutant animals (C). (D) A neuromast of a trilobite mutant animal is included for comparison. The planar polarity defects of hair cells in trilobite mutants are associated with the loss of Vangl2 function specifically in neuromasts, rather than a consequence of gastrulation defects. (Scale bars: 10 μm.) |