- Title
-
Analysis of axis induction mutant embryos reveals morphogenetic events associated with zebrafish yolk extension formation
- Authors
- Lyman Gingerich, J., Lindeman, R., Putiri, E., Stolzmann, K., and Pelegri, F.
- Source
- Full text @ Dev. Dyn.
|
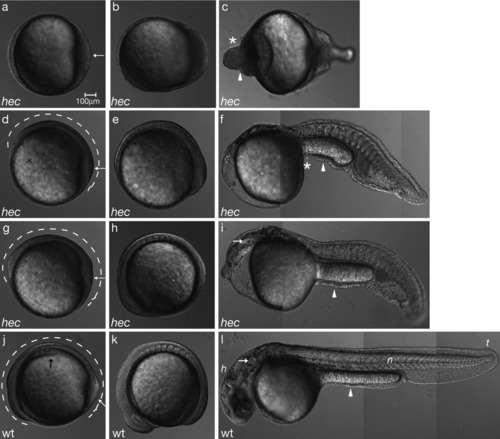
hec mutant embryos display morphogenesis defects consistent with the posteroventralization of cell fates. Radialized hec mutant embryos at the three-somite stage have an accumulation of cells at the tail bud (a, white arrow), whereas wild-type embryos already display a thickening of the dorsal side (j, black arrow) and elongation of the anterior-posterior axis (j, dotted line). The degree of enlargement of the posterior region and length of the anterior-posterior axis (a,d,g,j, white arrows and dotted lines, respectively) correlate with the phenotype at 24 hours postfertilization (hpf). a-c: During somitogenesis, radialized hec mutant embryos show an enlargement of the posterior tail bud followed by an accumulation of cells at the anterior end of the embryo (c, asterisk). d-f: Moderately affected mutants develop some dorsoanterior structures, but no head structures by 24 hpf. g-i: Mildly affected mutants develop more dorsoanterior structures, including posterior head structures such as the ear (white arrow in i), by 24 hpf. j-l: Wild-type embryos develop normal dorsoanterior structures, including a normal head and notochord by 24 hpf. (h, head; n, notochord; t, tail; white arrow in l: ear). Asterisks in c and f highlight cell accumulations in ventroposteriorized mutants. Arrowheads in c, f, i, and l point to constrictions in the embryos. Anterior, left; posterior, right; dorsal, top (when distinguishable). For each row, panels are still images from time-lapse microscopy analysis of the same embryo. |
|
hec mutant embryos display morphogenesis defects consistent with the posteroventralization of cell fates. Radialized hec mutant embryos at the three-somite stage have an accumulation of cells at the tail bud (a, white arrow), whereas wild-type embryos already display a thickening of the dorsal side (j, black arrow) and elongation of the anterior-posterior axis (j, dotted line). The degree of enlargement of the posterior region and length of the anterior-posterior axis (a,d,g,j, white arrows and dotted lines, respectively) correlate with the phenotype at 24 hours postfertilization (hpf). a-c: During somitogenesis, radialized hec mutant embryos show an enlargement of the posterior tail bud followed by an accumulation of cells at the anterior end of the embryo (c, asterisk). d-f: Moderately affected mutants develop some dorsoanterior structures, but no head structures by 24 hpf. g-i: Mildly affected mutants develop more dorsoanterior structures, including posterior head structures such as the ear (white arrow in i), by 24 hpf. j-l: Wild-type embryos develop normal dorsoanterior structures, including a normal head and notochord by 24 hpf. (h, head; n, notochord; t, tail; white arrow in l: ear). Asterisks in c and f highlight cell accumulations in ventroposteriorized mutants. Arrowheads in c, f, i, and l point to constrictions in the embryos. Anterior, left; posterior, right; dorsal, top (when distinguishable). For each row, panels are still images from time-lapse microscopy analysis of the same embryo. |
|
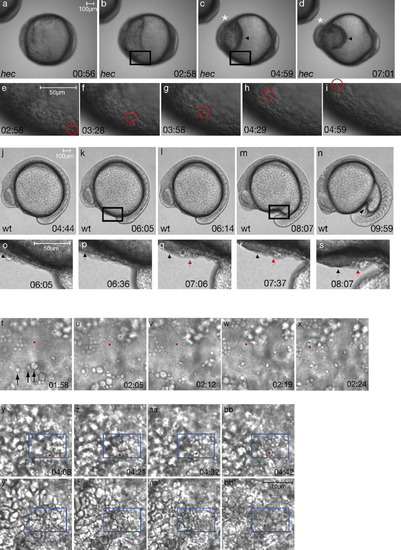
Cells accumulate in the ventral region of the yolk during the somitogenesis stages. a-d: Stills from Supplementary Movie S1 of a radialized hec mutant embryo. c,d: The asterisks indicate the cap of accumulating postmigratory cells; the arrows show anterior limit of yolk cell. e-i: Movement of one cell (marked with red dot) traced over time. e and i are magnifications of boxed areas in b and c, respectively. j-n: Stills from Supplementary Movie S3 of the beginning of yolk extension formation in a wild-type embryo. o-s: Region of yolk extension during the initiation of its formation. Accumulation of cells occurs over time in the region anterior to the tail bud (red arrow). Black arrow (o-s) indicates posteriorly moving population of anteriorly derived prechordal plate cells. o and s are magnifications of boxed areas in k and m, respectively. Time shown is time elapsed since the beginning of the recording, which corresponds to approximately the tail bud stage. Anterior, left; posterior, right; dorsal (when distinguishable), top. t-x: High-magnification imaging of the leading edge of the anteriorly migrating cells, when anteriorly migrating cells appear relatively disperse. The movement of one cell (green dot, slightly out of focus, as it is in a more internal plane) is shown in relation to an enveloping layer (EVL) cell (red dot) over the yolk of a hec mutant embryo. Stills are from Supplementary Movie S2a. The migrating cell, in a lower focal plane, approaches and then travels under the EVL cell. Note, however, that the EVL cells also undergo some anterior displacement, albeit not as pronounced as that of the migrating internal cells. Arrows indicate round structures of various sizes on the outer surface, which are often observed at the boundaries of EVL cells. y-bb′: High-magnification imaging of the same embryo as in t-x later in development, when the anteriorly migrating cells travel en masse. The movement of one cell (green dot in y-bb) is traced in relation to overlying cells (red dot in y′-bb′). y-bb: Stills are from Supplementary Movie S2b showing the outer surface focal plane. At this stage, the characteristic flattened EVL morphology is less recognizable. The red dot indicates a relatively stationary surface structure. y′-bb′: same fields of view as y-bb, but focusing on the underlying cellular layer of migrating cells. Time shown is time elapsed since the beginning of the recording at the tail bud stage. Anterior, left; posterior, right. Scale bar in bb′ refers to panels t-bb′. |
|
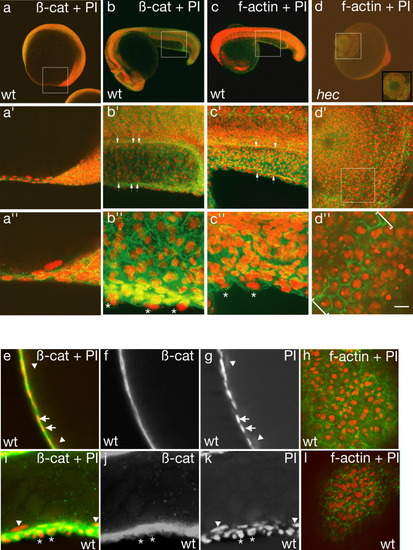
β-Catenin and f-actin localization reveal the presence of an extra layer of cells overlying the enveloping layer (EVL) in the yolk extension region. a,b,e-g,i-k: β-Catenin and propidium iodide labeling of a wild-type embryo. c,d,h,l: f-Actin and propidium iodide labeling of wild-type and hec mutant embryos. a′-d′,a″-d″: Progressively higher magnifications of a-d. Nuclei in the wild-type yolk extension tend to be aligned in rows in a dorsal to ventral direction (white arrows in b′, c′). White asterisks in b″ and c″ denote protruding cells. Site of constriction in radialized hec mutant embryos is shown by brackets in d″. Inset in d shows an optical section of the boxed area. e-h: Single optical sections of a wild-type yolk ball. e-g: Side views optical cross-sections showing the presence of a highly flattened epithelium. h: Surface view showing that the highly flattened layer consists of large, flattened cells with the characteristic EVL morphology. The presence of nuclei straddling boundaries between EVL cells indicates that there are additional cells underneath the EVL, although the appearance of the epithelium as a single cell layer in most optical cross-sections (as in e-g) indicates that these internal cells must have a highly flattened morphology. i-l: Single optical sections of a wild-type yolk extension. i-k: Optical cross-sections showing that the surface epithelium appears as multiple layers of cuboidal or rounded cells, which often protrude from the surface of the embryo. l: Surface view showing a surface layer of cells. The presence of additional nuclei straddling cell boundaries reflects the presence of multiple cellular layers, as shown by the side view (i-k). White asterisks in i-k indicate nuclei of overlying layer; white arrows in e, g indicate EVL nuclei, as identified from the surface position and the flattened structure of the cells; white arrowheads in e, g, l, and k indicate yolk syncytial layer (YSL) nuclei. Anterior, left; posterior, right; dorsal, top (when distinguishable). Green, β-catenin or f-actin; red, propidium iodide. Scale bar in d″ = 120 μm in a-d, 60 μm in a′-d′, 20 μm in a″-d″,e-l. |
|
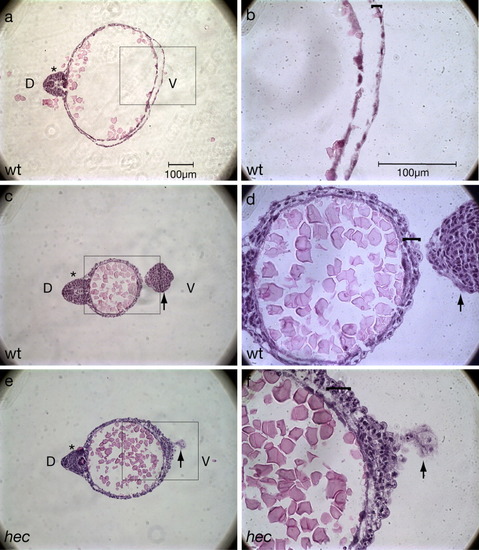
Histological sections confirm the presence of additional cell layers in the yolk extension region. a-f: Hematoxylin/eosin labeling of a wild-type embryo (a-d) and a hec mutant embryo with a rudimentary axis (e,f). b,d, and f are magnifications of the boxed areas in a, c, and e, respectively. a,b: Cross-section within the region of the yolk cell proper of a wild-type embryo at the 18-somite stage (18 hpf). The wild-type yolk cell is bounded by a highly flattened epithelium (bracket in b). c,d: Cross-section within the yolk extension region of the same wild-type embryo as in a,b. d: The wild-type yolk extension is surrounded by a surface consisting of multiple layers of less flattened and often protruding cells (bracket). e,f: Cross-section within the region of embryonic constriction of a partial axis hec mutant embryo at 18 hours postfertilization (hpf). f: Like the yolk extension in wild-type embryos, the ventral yolk surface of partially ventroposteriorized hec mutant embryo is bounded by a surface consisting of several cuboidal cells (bracket). This surface, however, typically consists of more layers than in the yolk extension region of wild-type embryos. The tail (indicated by a black arrow) curves downward at this stage so that it is also visible in sections c-f. In a,c, the embryonic axis (or rudimentary axis in e) is indicated with an asterisk, and "D" and "V" in a,c denote the dorsal and ventral regions, respectively (also indicated in the axis-deficient hec mutant embryo in e as deduced by the presence of the rudimentary dorsal axis). |
|
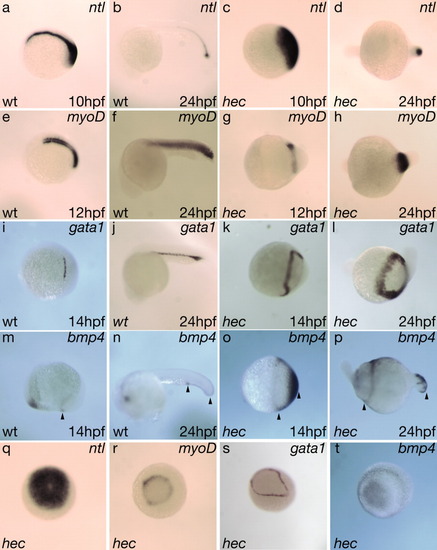
hec mutant embryos display morphogenesis defects consistent with the posteroventralization of cell fates. Radialized hec mutant embryos at the three-somite stage have an accumulation of cells at the tail bud (a, white arrow), whereas wild-type embryos already display a thickening of the dorsal side (j, black arrow) and elongation of the anterior-posterior axis (j, dotted line). The degree of enlargement of the posterior region and length of the anterior-posterior axis (a,d,g,j, white arrows and dotted lines, respectively) correlate with the phenotype at 24 hours postfertilization (hpf). a-c: During somitogenesis, radialized hec mutant embryos show an enlargement of the posterior tail bud followed by an accumulation of cells at the anterior end of the embryo (c, asterisk). d-f: Moderately affected mutants develop some dorsoanterior structures, but no head structures by 24 hpf. g-i: Mildly affected mutants develop more dorsoanterior structures, including posterior head structures such as the ear (white arrow in i), by 24 hpf. j-l: Wild-type embryos develop normal dorsoanterior structures, including a normal head and notochord by 24 hpf. (h, head; n, notochord; t, tail; white arrow in l: ear). Asterisks in c and f highlight cell accumulations in ventroposteriorized mutants. Arrowheads in c, f, i, and l point to constrictions in the embryos. Anterior, left; posterior, right; dorsal, top (when distinguishable). For each row, panels are still images from time-lapse microscopy analysis of the same embryo. EXPRESSION / LABELING:
|