- Title
-
The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity
- Authors
- Barresi, M.J., Stickney, H.L., and Devoto, S.H.
- Source
- Full text @ Development
|
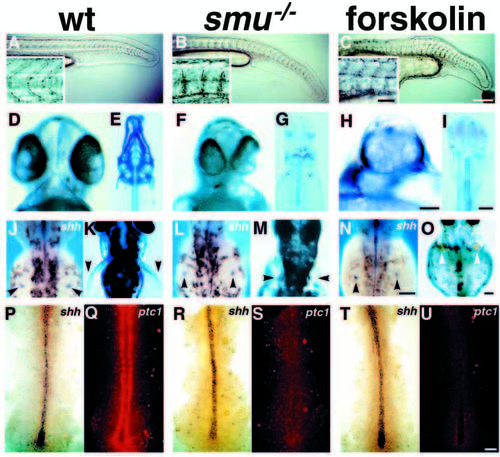
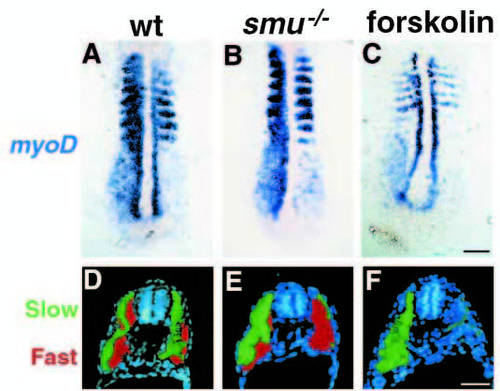
smu mutant phenotypes resemble those seen in forskolin treated embryos. (A-C) Side views of 24h live embryos; insets show higher magnification views of somite morphology. Wild-type embryos (A) have typical chevron-shaped somites while smu-/- (B) and forskolin-treated (C) embryos have blocky, U-shaped somites and ventrally curled tails. (D,F,H) Ventral views of live 48h embryos. The eyes in wild-type embryos (D) are separated, whereas smu-/- (F) and forskolin-treated (H) embryos have partial cyclopia. (E,G,I) Ventral views of Alcian Bluestained 3.5d embryos. Jaw, head, fin and trunk cartilage is well developed in wild-type embryos (E), while smu-/- (G) and forskolin-treated (I) embryos have a nearly complete loss of jaw and head cartilage. Trunk cartilage is similar in wild-type, smu-/- and forskolin-treated embryos. (J,L,N) Dorsal views of 36h embryos labeled by in situ hybridization for shh. The fin buds (arrowheads) of wild-type (J), smu-/- (L), and forskolin-treated (N) embryos all express shh in a similar pattern. (K,M,O) Dorsal views of live 3d embryos. Pectoral fins (arrowheads) are well developed in wild-type embryos (K), whereas pectoral fins are severely reduced in smu-/- (M) and forskolin-treated (O) embryos. (P-U) Dorsal views of shh (blue) and ptc1 (red) expression in 12h embryos. A white light (P,R,T) and a fluorescence (Q,S,U) image of each embryo is shown. Wild-type (P), smu-/- (R), and forskolin-treated (T) embryos express shh similarly in the notochord. Wild-type (P,Q) embryos express high levels of ptc1 in paraxial mesoderm cells adjacent to the notochord. smu-/- (R,S) and forskolin-treated (T,U) embryos do not express detectable ptc1 in the paraxial mesoderm. All images are oriented such that anterior is to the left (side views) or to the top (dorsal and ventral views). Bars: 200 μm (A-C); 50 μm (A-C insets); 50 μm (D,F,H); 100 μm (E,G,I); 100 μm (J,L,N); 100 μm (K,M,O); 50 μm (P-U). EXPRESSION / LABELING:
PHENOTYPE:
|
|
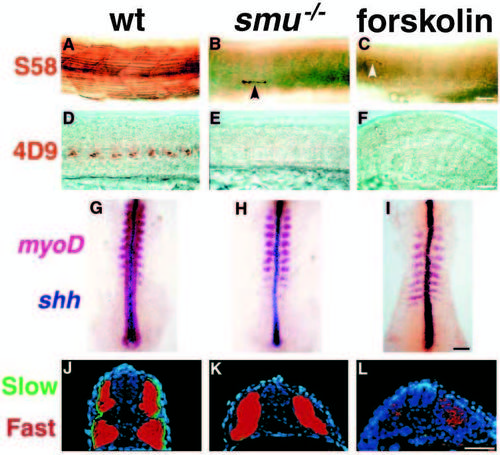
smu-/- and forskolin-treated embryos have defects in slow muscle fiber type development. (A-C) Side views of S58 antibody labeled 24h embryos. S58 specifically labels slow muscle fibers. In wild-type embryos (A) approximately 20 slow muscle fibers span each chevron-shaped somite very clearly. In smu-/- (B) and forskolin-treated (C) embryos, slow muscle is markedly reduced; individual slow muscle fibers are marked with arrowheads. (D-F) Side views of 4D9 Engrailed antibody labeling of muscle pioneer slow muscle fibers at 24h. 4D9 labels 2-5 muscle pioneer nuclei per somite in wild-type (D), while smu-/- (E) and forskolin-treated (F) embryos have a complete loss of muscle pioneer slow muscle cells. (G-I) Dorsal view of 12h-14h embryos hybridized to show myoD (red) and shh (dark blue) expression. MyoD is expressed in wildtype embryos (G) in the somites and in the adaxial cells of the presomitic mesoderm. smu-/- (H) and forskolintreated (I) embryos express myoD in the somites, but do not have any expression in cells adjacent to the notochord. (J-L) Transverse sections through the trunk of 30h embryos labeled with S58 (green) and zm4 (red) monoclonal antibodies to identify slow and fast muscle fibers, respectively. In sections of wild-type embryos (J), slow muscle fibers form a monolayer around the deeper fast muscle fibers. smu-/- (K) and forskolin-treated (L) embryos have a nearly complete loss of S58 labeling throughout the entire trunk, whereas zm4 staining is still present. Bars, 50 μm (A-F); 50 μm (G-I); 100 μm (J-L). EXPRESSION / LABELING:
|
|
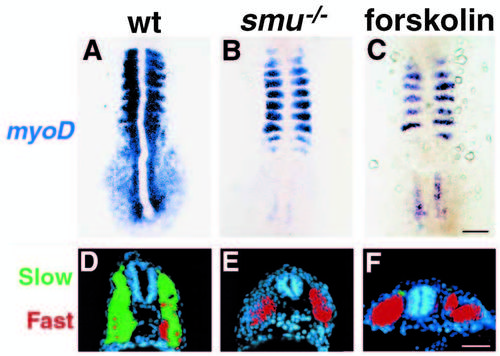
Shh overexpression in smu-/- and forskolin-treated embryos does not rescue slow muscle development. (A-C) shh mRNA injected embryos were fixed at 12h and labeled by in situ hybridization for myoD; dorsal views are shown. In 3/4 of the embryos from a cross of 2 smu heterozygotes (60/86), myoD expression is expanded into the segmental plate as a result of Shh overexpression (A). In 1/4 of the embryos (26/86), myoD expression is not affected by Shh overexpression (B). 100% of the forskolin-treated embryos failed to respond to Shh overexpression (C). (D-F) shh mRNA injected embryos were fixed at 30h and transverse sections were labeled for S58 (green) and zm4 (red). In wild-type embryos (D), most of the zm4-labeled fast muscle (red) is replaced by S58-labeled slow muscle (green), compare to Fig. 2J. Slow muscle is not rescued by Shh overexpression in smu-/- (E) or in forskolin-treated (F) embryos; compare to Fig. 2K,L. See also Fig. 4. Bars, 100 μm (A-C); 50 μm (D-F). |
|
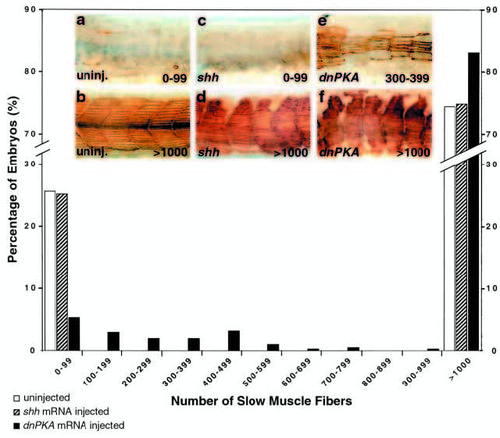
Quantitative analysis of slow muscle fiber numbers in uninjected, shh injected and dnPKA injected embryos from a cross of two smu+/- animals. S58- labeled slow muscle fibers in uninjected and injected embryos were counted blind, and fiber tallies binned in sets of 100 for clarity of presentation. Uninjected siblings of shh injected, dnPKA injected and rsmo injected embryos (Fig. 7) were pooled. shh injection (n=453) has no effect on the expected Mendelian proportion of mutant embryos; as in uninjected embryos (n=1219), approximately 25% had <100 fibers, and approximately 75% had >1000 fibers. Conversely, only 5% of the embryos injected with dnPKA exhibited <100 fibers (black bars, n=421). Numbers of dead and malformed embryos (in which we did not count fibers) for injected embryos and their siblings were very similar (shh injected: 58 of 511, siblings 34 of 607; dnPKA injected 10 of 431, siblings 29 of 651). Although we saw variation between clutches of uninjected embryos in the number of slow muscle fibers per embryo, among embryos with <1000 slow muscle fibers shh injection did not affect the number of fibers within clutches, whereas dnPKA injection consistently increased the mean number of slow muscle fibers. shh injected embryos had an average of 3.9±0.14 slow muscle fibers per fish (n=114), and their uninjected siblings had an average of 3.2±0.3 (n=154). This difference is not significant (t-test, P≤0.10). dnPKA injected embryos had an average of 259.0±25.6 slow muscle fibers per fish (n=71), while their uninjected siblings had an average of 8.3±0.6 (n=162). This difference is significant (t-test, P≤0.0005). Values are means ± s.e.m. Insets depict representative embryos for uninjected controls with 0-99 (a) and >1000 (b) fibers, shh injected embryos with 0-99 (c) and >1000 (d) fibers, and dnPKA injected embryos with 300-399 (e) and >1000 (f) fibers. |
|
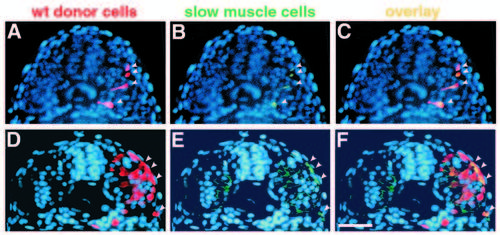
Transplanted wild-type muscle cells rescue slow muscle development in smu-/-. Transverse sections from two different smu-/- embryos show donor-derived, wild-type muscle cells. Sections were labeled with the F59 antibody, detected with a fluorescein-conjugated secondary antibody, and counterstained with Hoechst 33258 (blue). (A,D) Rhodamine-labeled wild-type cells (red, arrowheads). (B,E) F59-labeled slow muscle fibers (green, arrowheads). (C,F) merged micrographs. In A-C, four transplanted wild-type cells have developed into slow muscle fibers in an approx. 22h smu-/- host (C, yellow, arrowheads). Cells are still migrating through the somite. In D-F, transplanted wild-type cells have differentiated into both slow (4 cells; F, yellow, arrowheads) and fast (approx. 10 cells) muscle fibers in a 24h smu-/- host. Slow fibers can be distinguished from fast on the basis of the intensity of F59 labeling. Bar, 50 μm. |
|
dnPKA overexpression in smu-/- and forskolin-treated embryos rescues slow muscle development. dnPKA mRNA was injected into forskolin-treated embryos and embryos derived from a cross of two animals heterozygous for smu mutations. (A-C) Dorsal view of embryos fixed at 12h and labeled by in situ hybridization for myoD. (A) In the majority of embryos (187/194), myoD expression is expanded into the segmental plate as a result of dnPKA overexpression. (B) In some of these embryos, myoD labeling on one side of the embryo shows the same pattern as injected wild-type embryos, whereas the labeling on the other side resembles uninjected smu mutant embryos. We interpret these as mutant embryos in which the dnPKA mRNA was effective only on one side of the embryo. (C) Forskolin-treated embryos also respond to dnPKA mRNA. (D-F) dnPKA mRNA injected embryos fixed at 30h and labeled for S58 (green) and zm4 (red). (D) In wild-type embryos most of the zm4-labeled fast muscle (red) is replaced by S58-labeled slow muscle (green), compare to Fig. 2J (see also Du et al., 1997). (E) Slow muscle is on occasion rescued by dnPKA overexpression only on one side (compare to Fig. 2K); we interpret these as smu-/- embryos. (F) dnPKA overexpression rescues forskolin-treated embryos; compare to Fig. 2L. See also Fig. 4. Bars, 100 μm (A-C); 50 μm (D-F). |
|
Smoothened overexpression rescues slow muscle development in smu mutants. A plasmid encoding rat smoothened cDNA under the control of the CMV promoter was injected into embryos derived from a cross of two animals heterozygous for smu mutations. S58-labeled slow muscle fibers in uninjected and injected embryos were counted blind, and fiber tallies binned in sets of 50 for clarity of presentation. Uninjected siblings of shh injected and dnPKA injected embryos (Fig. 4) and rsmo injected embryos were pooled. Uninjected embryos (white bars, n=1219) displayed the expected Mendelian proportions: approx. 25% had <50 fibers, approx. 75% had >1000 fibers. rsmo overexpression resulted in a dramatic decrease in the percentage of embryos with <50 fibers and a consequent increase in the percentage of embryos with >50 fibers (black bars, n=351). Numbers of dead and malformed embryos (in which we did not count fibers) for injected embryos and their siblings were very similar (rsmo injected: 71 of 422, siblings: 80 of 492). Therefore we conclude that rsmo is not increasing the number of dead or deformed embryos. Among the embryos with <1000 slow muscle fibers, rsmo injected embryos had an average of 210.0±12.7 slow muscle fibers (n=96) and their uninjected siblings had an average of 22.0±1.3 (n=98). This difference is significant (t-test, P≤0.0005). Values are means ± s.e.m. Insets depict representative embryos for uninjected controls with 0-49 (a) fibers and rsmo injections with 200-249 (b) and >1000 (c) fibers. |