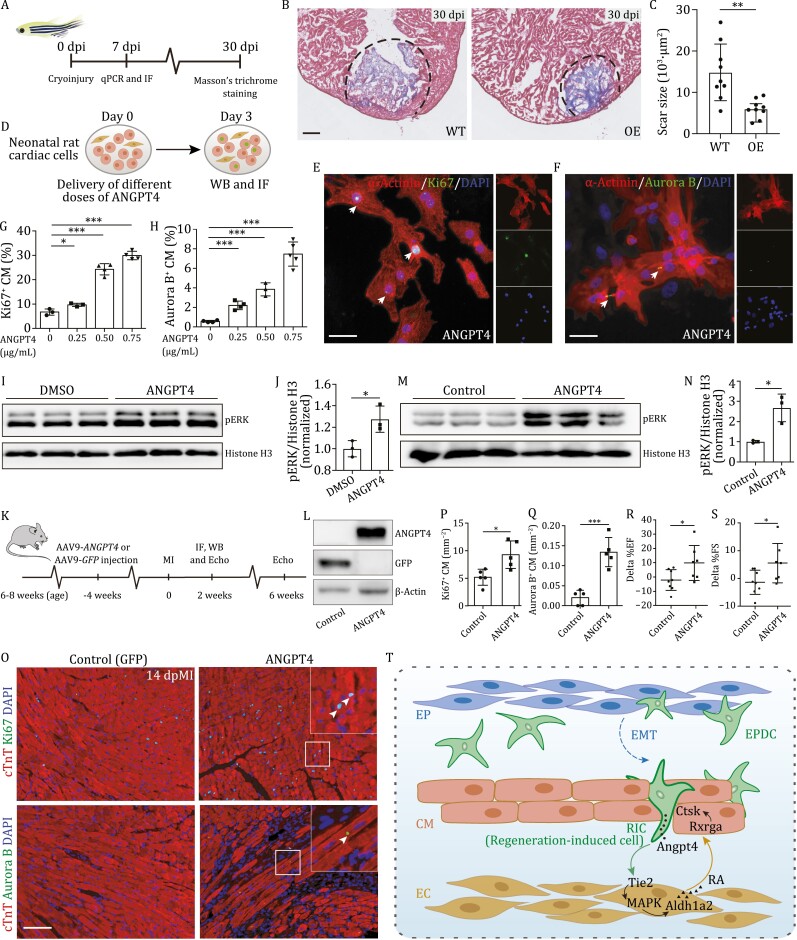
Figure 6.
ANGPT4 promotes cardiac repair in vitro and in vivo. (A) Experimental design to evaluate heart regeneration potential in angpt4 overexpressing zebrafish. (B) Masson?s trichrome staining results of adult zebrafish heart sections from wildtype (WT) and Tg(cmlc2:EGFP-angpt4) (OE) at 30 dpi. Red, muscle cells stained with acid fuchsin; blue, collagen stained with aniline blue. Scale bar, 100 ?m. (C) Statistical analysis results of scar size in 30 dpi of WT and Tg(cmlc2:EGFP-angpt4) (OE) hearts. Two-tailed Student?s t-test, **P < 0.01. n = 9 for each group. Error bar represents standard deviation. (D) Experimental design for treatment with different doses of recombinant ANGPT4 protein and studying its effect on the expression of cell cycle markers in NRCM. (E and F) Immunofluorescence staining results showing Ki67 (E) and Aurora B (F) signals in NRCM following treatment with 0.75 ?g/mL recombinant human ANGPT4 protein. Scale bar, 25 ?m. (G and H) Statistical analysis of Ki67 and Aurora B positive NRCM in (E and F). Two-tailed Student?s t-test, *P < 0.05; ***P < 0.001. n = 3?5 for each group. Error bar represents standard deviation. (I) Western blot of pERK in cultured primary neonatal rat cardiac cells with or without 0.75 ?g/mL ANGPT4 protein treatment. (J) Statistical analysis of (I) showing pERK signals were significantly increased in 0.75 ?g/mL ANGPT4 treated cardiac cells. Vertical axis shows the pERK-to-Histone H3 ratio, normalized to the control group. Student?s t-test, *P < 0.05. n = 3 for each group. Error bar represents standard deviation. (K) Experimental timeline for evaluation of cardiomyocyte proliferation and cardiac function after MI in mice injected with AAV9-GFP and AAV9-ANGPT4. (L) Western blot analysis showing the expression of GFP or ANGPT4 3 weeks after intravenous AAV9-GFP or AAV9-ANGPT4 injection. (M) Western blot analysis showing the activation of pERK at 2 weeks post MI in AAV9-ANGPT4 injected mouse hearts. (N) Statistical analysis of (M), showing pERK signals were significantly increased in AAV9-ANGPT4 injected mice relative to AAV9-GFP injected mice. Vertical axis shows the pERK-to-Histone H3 ratios, normalized to the control group. Student?s t-test, *P < 0.05. n = 3 for each group. Error bar represents standard deviation. (O) Immunofluorescence staining showing Ki67 and Aurora B signals at 2 weeks post MI in cardiomyocytes of AAV9-GFP and AAV9-ANGPT4 injected mice. Scale bar, 100 ?m. (P and Q) Statistical analysis of Ki67 and Aurora B positive cardiomyocyte in (M). Two-tailed Student?s t-test, *P < 0.05; ***P < 0.001. n = 5 for each group. Error bar represents standard deviation. (R and S) Statistical analysis showing differences of cardiac function indexed by EF (%) and FS (%) measured by echocardiography between 2 weeks and 6 weeks post MI. Two-tailed Student?s t-test, *P < 0.05. n = 7?8 for each group. Error bar represents standard deviation. (T) Model depicting an Angpt4-mediated cellular coordination network among major cardiac cell types during zebrafish heart regeneration. Angpt4 is specifically activated and required for heart regeneration in both zebrafish and mammals. This model illustrates the underlying molecular and cellular mechanisms, where Angpt4 initiates a cellular coordination cascade along an EPDC (RIC)-EC-CM axis by activating MAPK-RA signaling pathways during heart regeneration.