Fig 2
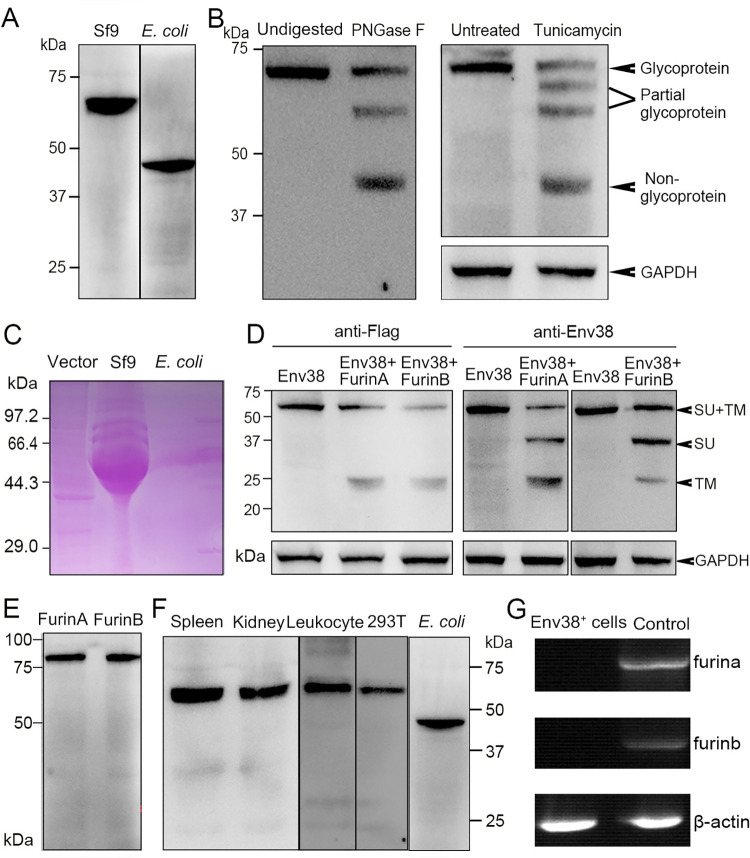
(A) Western blot analysis for the expression of Env38 protein with mouse anti-His Ab (1:5,000). The Env38 proteins expressed in eukaryotic Sf9 cells and prokaryotic E. coli cells were determined to be approximately 60 kDa and 48 kDa, respectively. (B) Western blot analysis for N-glycosylation of Env38 protein with treatment of PNGase F and tunicamycin, in which the Env38 protein derived from Sf9 cells was treated with PNGase F (500 U/ml), and the Env38-expressing HEK293T cells transfected with pcDNA3.1-Flag-Env38-LTR (0.6 ?g/ml) was treated with tunicamycin (5 ?g/ml). The Env38 protein was separated by 12% SDS-PAGE. Env38 protein derived from Sf9 cells without digestion and HEK293T cells without treatment were served as controls. GAPDH was used as a loading control. (C) Detection of the glycosylation of Env38 protein by PAS reaction. The Env38 proteins were expressed in Sf9 cells or E. coli cells, and separated by 12% SDS-PAGE followed by staining with Schiff reagent. Env38 protein derived from Sf9 cells exhibited a strong positive staining, while Env38 protein derived from E. coli cells showed no staining. (D) Western blot analysis for the detection of enzymatic cleavage of Env38 protein by Furins. Coexpression of Env38 protein (with a Flag-tag at C-terminus) and FurinA or FurinB in HEK293T cells resulted in cleavage of Env38 with two protein bands corresponding to the intact Env38 protein (60 kDa) and TM subunit (25 kDa) when detected with anti-Flag Ab, and three protein bands corresponding to the intact Env38 protein (60 kDa), SU subunit (35 kDa) and TM subunit (25 kDa) when detected with mouse anti-Env38 Ab. GAPDH was used as a loading control. (E) Western blot analysis for the expression of FurinA and FurinB proteins in HEK293T cells. (F) Western blot analysis for the reactivity and specificity of mouse anti-Env38 polyclonal Ab. The anti-Env38 Ab clearly combined with the target natural Env38 proteins derived from spleen, head kidney and leukocytes, as well as recombinant Env38 proteins from HEK293T and E. coli cells with molecular weights of 60 kDa and 48 kDa, respectively. (G) Detection of the transcriptional expression of furina and furinb genes in Env38+ cells sorted from leukocytes from spleen, head kidney and peripheral blood of zebrafish stimulated with SVCV (105 TCID50) using RT-PCR. Total leukocytes were used as a control.