Fig. 4
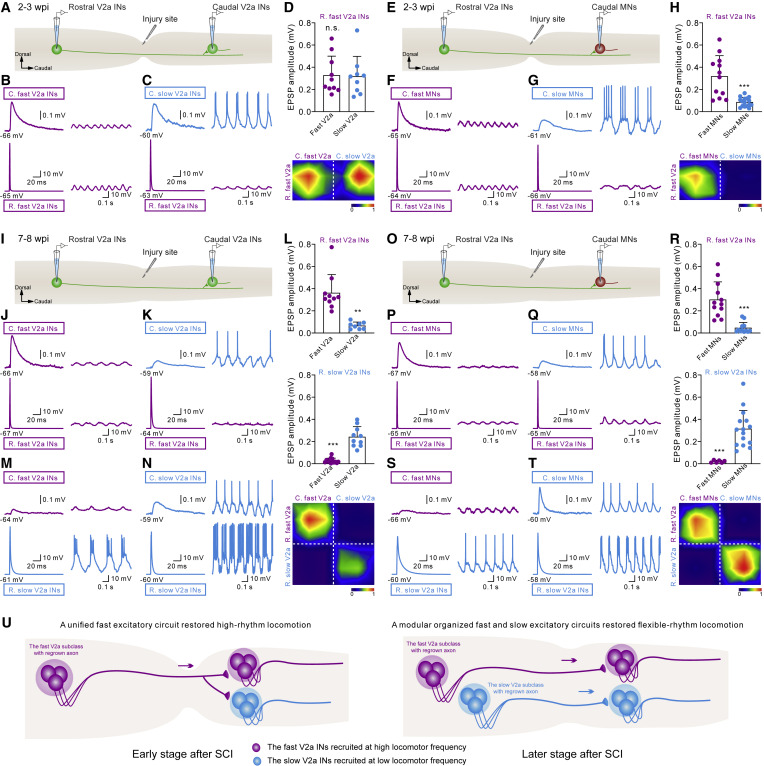
Figure 4. The orderly reestablishment of fast and slow excitatory V2a circuit modules underlies stepwise restoration of locomotor function (A, E, I, and O) Illustration of paired whole-cell patch-clamp recording between a V2a interneuron rostral to the lesion with regrown axons and a V2a interneuron (A and I) or a motor neuron (E and O) caudal to the lesion. (B, C, F, and G) Paired recordings showing a monosynaptic connection between a rostral fast V2a interneuron and a fast (B) or a slow (C) V2a interneuron or a fast (F) or a slow (G) motor neuron caudal to the lesion at 2?3 wpi. (D and H) Statistical analysis and connectivity matrix of a rostral fast V2a interneuron induced EPSPs in a fast or a slow V2a interneuron (D), or a fast or a slow motor neuron (H) caudal to the lesion at 2?3 wpi. (J and K and P and Q) Paired recordings showing a monosynaptic connection between a rostral fast V2a interneuron with regrown axons and a fast (J) or a slow (K) V2a interneuron or a fast (P) or a slow (Q) motor neuron caudal to the lesion at 7?8 wpi. (M and N and S and T) Paired recordings showing monosynaptic connections between a rostral slow V2a interneuron with regrown axons and a fast (M) or a slow (N) V2a interneuron or a fast (S) or a slow (T) motor neuron caudal to the lesion at 7?8 wpi. (L and R) Statistical analysis and connectivity matrix of a rostral fast/slow V2a interneuron induced EPSPs in a fast or a slow V2a interneuron (L), a fast or a slow motor neuron (R) caudal to the lesion at 7?8 wpi, respectively. (U) Proposed model of the reorganization of intraspinal excitatory circuits dominated by a V2a interneuron with regrown axons that underpins the restoration of locomotor rhythm after SCI. All data shown are mean ± SD. ???p < 0.001, significant difference. One dot equals one neuron, and only one pair of neurons was recorded from each fish. See also Figure S4.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Rep.