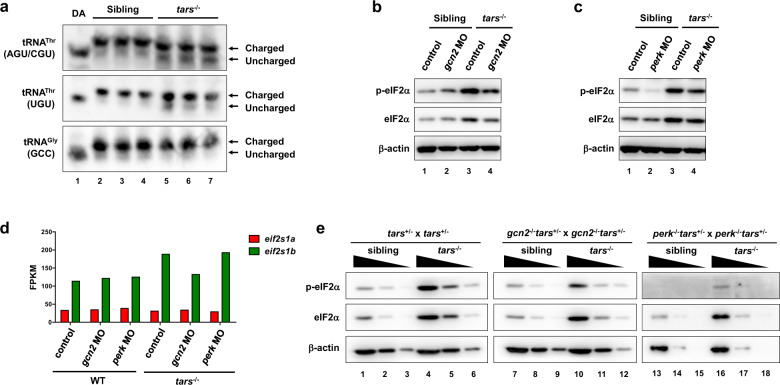
Fig. 3
a Northern blot results showing the increased uncharged tRNAThr in the tars−/− embryos compared with the siblings. Charged and uncharged tRNAs (upper and lower bands, respectively) were separated in an acid polyacrylamide/urea gel system; the three types of tRNAThr were hybridized with specific probes that could recognize tRNAThr(AGU/CGU) or tRNAThr(UGU). The tRNAGly(GCC) was used as a negative control. Deacylated tRNAs (DA) were used to mark the migration position of the uncharged tRNAs on the gels. b, c Immunoblot analysis of the phosphorylated eIF2α (p-eIF2α) and total eIF2α upon morpholino-mediated knockdown of Gcn2 or Perk in the WT and tars−/− embryos. β-actin was used as the loading control. Note that gcn2 MO and perk MO reduced the phosphorylated eIF2α in the tars−/− embryos to a comparable extent, whereas only perk MO reduced the eIF2α phosphorylation in the WT embryos. d Expression levels of the mRNAs of the two zebrafish eIF2α-coding genes, eif2s1a and eif2s1b, in the embryos of indicated genotypes. Presented are fragments per kilobase per million mapped reads (FPKM) values from RNA-seq data. Note that eif2s1b is upregulated in the tars−/− embryos, which explains the accordingly increased protein levels as indicated by the immunoblot results. e Immunoblot analysis of the phosphorylated and total eIF2α level in the gene knockout embryos, which were produced by crossing the indicated mutant lines. Each sample was loaded in a 3-fold serial dilution to facilitate quantification. Note that the basal level of p-eIF2α was hardly detectable in the perk−/− “normal” (sibling) embryos (lanes 13–15), and that the p-eIF2α in the gcn2−/−tars−/− embryos was decreased, but not eliminated, compared with the tars−/− embryos (compare lanes 7–12 with 1–6).