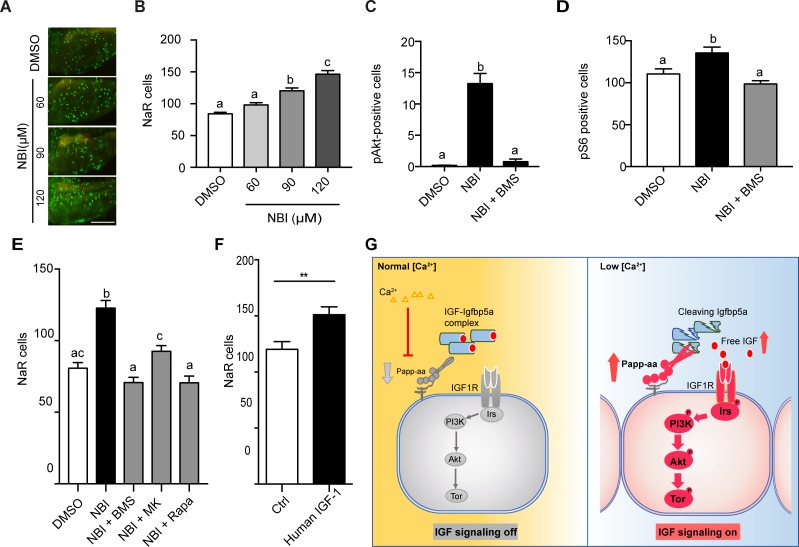
Figure 6
Disruption the IGF/Igfbp complex activates IGF-Akt-Tor signaling and promotes NaR cell reactivation.
(A–B) Tg(igfbp5a:GFP) fish were transferred to normal [Ca2+] medium containing the indicated doses of NBI-31772 at three dpf. Two days later, NaR cells were quantified. Representative images are shown in (A) and quantified data in (B). Scale bar = 0.2 mm. n = 25 ~ 27 fish/group. (C) Wild-type fish were treated with 90 µM NBI-31772 with or without 0.3 µM BMS-754807 from 3 to 4 dpf. The number of cells positive for phosphorylated Akt staining were quantified and shown. n = 19 ~ 23 fish/group. (D) Larvae treated as described in (C) were stained for phosph-S6 and quantified. n = 14 ~ 15 fish/group. (E) Tg(igfbp5a:GFP) fish were treated with NBI-31772 (90 µM) together with BMS-754807 (0.3 µM), MK2206 (8 µM), or Rapamycin (5 µM) from 3 to 5 dpf. NaR cell number was quantified and shown. n = 10 ~ 24 fish/group. (F) Tg(igfbp5a:GFP) fish were treated with human IGF1 (150 ng/ml) in E3 embryo medium from 3 to 5 dpf. NaR cells were quantified and shown. n = 35 ~ 36 fish/group. **p<0.01, unpaired t-test. (G) Proposed model of Papp-aa function as a [Ca2+]-regulated molecular switch of IGF signaling in epithelial cells. Left panel: under normal [Ca2+] conditions, Papp-aa proteolysis activity is suppressed. Igfbp5a is intact and it inhibits IGF signaling by binding to IGFs and prevents their binding to the IGF1 receptor. The IGF- PI3 kinase-Akt-Tor signaling is inhibited in NaR cells. Right panel: under low [Ca2+] conditions, Papp-aa activity is increased. This increases Igfbp5a proteolytic cleavage and releases IGFs from the Igfbp5a/IGF complex. Bioavailable IGFs binds to IGF1 receptor and activates PI3 kinase-Akt-Tor signaling in NaR cells and promotes their reactivation and proliferate.